Synthesis method of moxifloxacin hydrochloride impurity
A technology of moxifloxacin hydrochloride and its synthesis method, which is applied in the field of medicinal chemistry, can solve problems such as the synthesis method not mentioned, and achieve the effect of simple and easy operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a) In the reaction flask, add 10 g of moxifloxacin hydrochloride (purchased from Suzhou Langke Technology Co., Ltd.), 100 ml of 90% (w / w) formic acid and 80 ml of 35% (w / w) formaldehyde solution, and stir at 95°C Reflux the reaction until complete (about 4h), cool to room temperature, and concentrate the reaction solution to dryness under reduced pressure, add 50ml of water to wash with water, then concentrate to dryness under reduced pressure; add 50ml of water to the obtained residue, heat and stir to dissolve, add an appropriate amount of activated carbon (0.5 g), stirred for 30 min, filtered, the filtrate was adjusted to pH = 7 with 5% (w / w) sodium hydroxide solution, crystals were precipitated, filtered, washed with water, and dried at 60°C to obtain 9 g of the intermediate;
[0032] b) Take 9g of the intermediate and place it in a beaker, add 50ml of ethanol, heat to dissolve, adjust its pH=2 with 10% (v / v) hydrochloric acid, then add an appropriate amount of activ...
Embodiment 2
[0041]1. Preparation of moxifloxacin hydrochloride
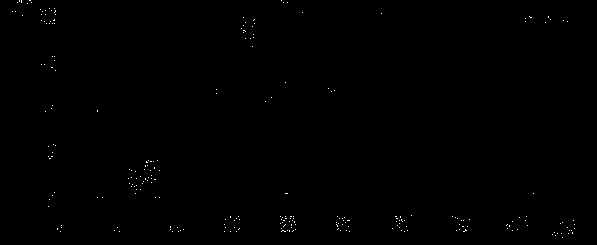
[0042] 1a) 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 , O 4 -Synthesis of boron diacetate
[0043] The synthetic route is as follows:
[0044]
[0045] In a 500ml reaction bottle, add 140g of acetic anhydride, 0.4g of zinc chloride, and 25g of boric acid in sequence, stir evenly, and slowly heat up to 35-40°C. After the reaction, the temperature rises to 110±2°C, reacts for 1.5h, and then cools down to 60~70°C, add 80g 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid ethyl ester, then heat up to React at 85-95°C for 3 hours, TLC shows a product spot, cool down to 40°C, slowly add the reaction solution into 700 g of ice water, stir for 60 minutes, filter, rinse the filter cake with ice water, and dry at 40°C , to obtain 100g of light yellow solid, melting point: 110~116℃;
[0046] 1b) 1-cyclopropyl-7-[(S,S)-2,8-diazo-bicyclo[4.3.0]nonan-8-yl]-...
Embodiment 3
[0062] Embodiment 3 (synthetic impurity with moxifloxacin)
[0063] a) In the reaction flask, add 10 g of moxifloxacin (purchased from Suzhou Langke Technology Co., Ltd.), 70 ml of 85% (w / w) formic acid and 34 ml of 40% (w / w) formaldehyde solution, and stir and reflux at 90 ° C. to complete (about 4.5h), cooled to room temperature, the reaction solution was concentrated to dryness under reduced pressure, added 50ml of water to wash, and then concentrated to dryness under reduced pressure; the resulting residue was added to 50ml of water, heated and stirred to dissolve, and an appropriate amount of activated carbon (0.4g ), stirred for 20 min, filtered, and the filtrate was adjusted to pH=7 with 10% (w / w) sodium hydroxide solution, crystals were precipitated, filtered, washed with water, and dried at 70°C to obtain 8 g of the intermediate;
[0064] b) Put the intermediate obtained in step a) into a beaker, add 50ml of methanol, heat to dissolve, adjust its pH=4 with 5% (v / v) hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com