Novel antidiabetic medicine
An anti-diabetic and drug technology, applied in the field of new chemical drugs and their design, can solve the problems of low drug action intensity, and achieve the effects of not easy to degrade, low cost, and simple drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
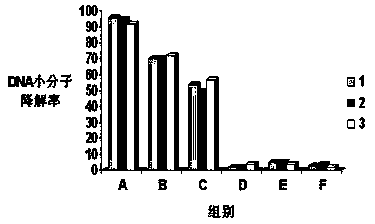
[0033] Embodiment 1 Determination of the optimal lasso structure
[0034] By culturing DNA molecules of various drug candidate compounds in the immortal cell line HEK293 for 1 week, and then measuring the degradation rate of DNA molecules of various drug compounds, that is, 100% degradation rate means complete degradation, 0% degradation rate means no degradation, The lower the degradation rate of the DNA molecule of the drug compound, the more the structure of the drug compound can resist the degradation of various nucleases, the stronger the degradation ability, and the more stable the structure.
[0035] Among them, (1) CCCATCTCTGGTGCC, (2) CGGCCACACACCAT, (3) CACGGGTGCAAACAG were divided into 6 experimental groups according to the modified structure, which were recorded as A, B, C, D, E, F, and the modification method was selected from both ends of the methyl group. The specific modification structure is shown in Table 1.
[0036] test group modified struct...
Embodiment 2
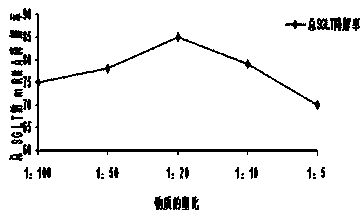
[0038] Example 2 Determination of the ratio of lead compound to modified compound
[0039] For the following compound CACGGGTGCAAACAG (referred to as compound A), the chemical modification of methylation at both ends was carried out according to 5 modified bases-5 unmodified bases-5 modified bases (referred to as compound B ), mix compounds A and B in 5 different ratios. Specifically, 10 μM of Compound B modified with the optimal lasso structure was added to each group, and then each group was added with different concentrations of chemically modified Compound A, the concentrations were 0.1 μM, 0.2 μM, 0.5 μM, 1 μM, 2 μM , so the ratio of A:B is 1:100, 1:50, 1:20, 1:10 and 1:5. The above-mentioned groups were mixed and cultured in the kidney cell line MDCK for 2 weeks, and then the contents of SGLT1 and SGLT2 were detected by RT-PCR method, and the results were shown by the efficiency of inhibiting the mRNA of total SGLT.
[0040] The result is as figure 2 As shown, the ...
Embodiment 3
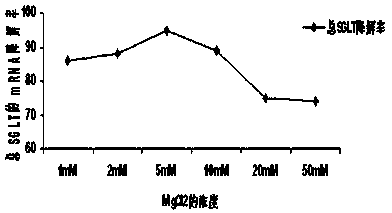
[0041] Example 3 Best MgCl 2 determination of concentration
[0042] For the following compound CACGGGTGCAAACAG (referred to as compound A), the chemical modification of methylation at both ends was carried out according to 5 modified bases-5 unmodified bases-5 modified bases (referred to as compound B ), mixed according to the optimal ratio of 1:20 (A:B), and then dissolved in 1mM, 2mM, 5mM, 10mM, 20mM and 50mM MgCl 2 After that, the mixed cells were cultured in the kidney cell line MDCK for 2 weeks, and then the contents of SGLT1 and SGLT2 were detected by RT-PCR method, and the results were shown by the efficiency of inhibiting the mRNA of total SGLT.
[0043] The result is as image 3 As shown, the drug is in 1mM, 2mM, 5mM, 10mM, 20mM and 50mM MgCl 2 The inhibition efficiencies in the solvents were 86%, 88%, 95%, 89%, 75%, 74% and 72%, respectively, and the higher the inhibition efficiency, the higher the potency of the drug. From the results it can be seen that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com