Hydrometallurgical method for separating and extracting Mn, Fe, Pb and Ag from silver-manganese ore
A technology of hydrometallurgy and silver-manganese ore, which is applied in the hydrometallurgy field of Fe, separation and extraction of Mn, Pb and Ag, can solve the problems of low recovery rate, complicated environmental management, high energy consumption, etc., and achieve low cost and environmental governance easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
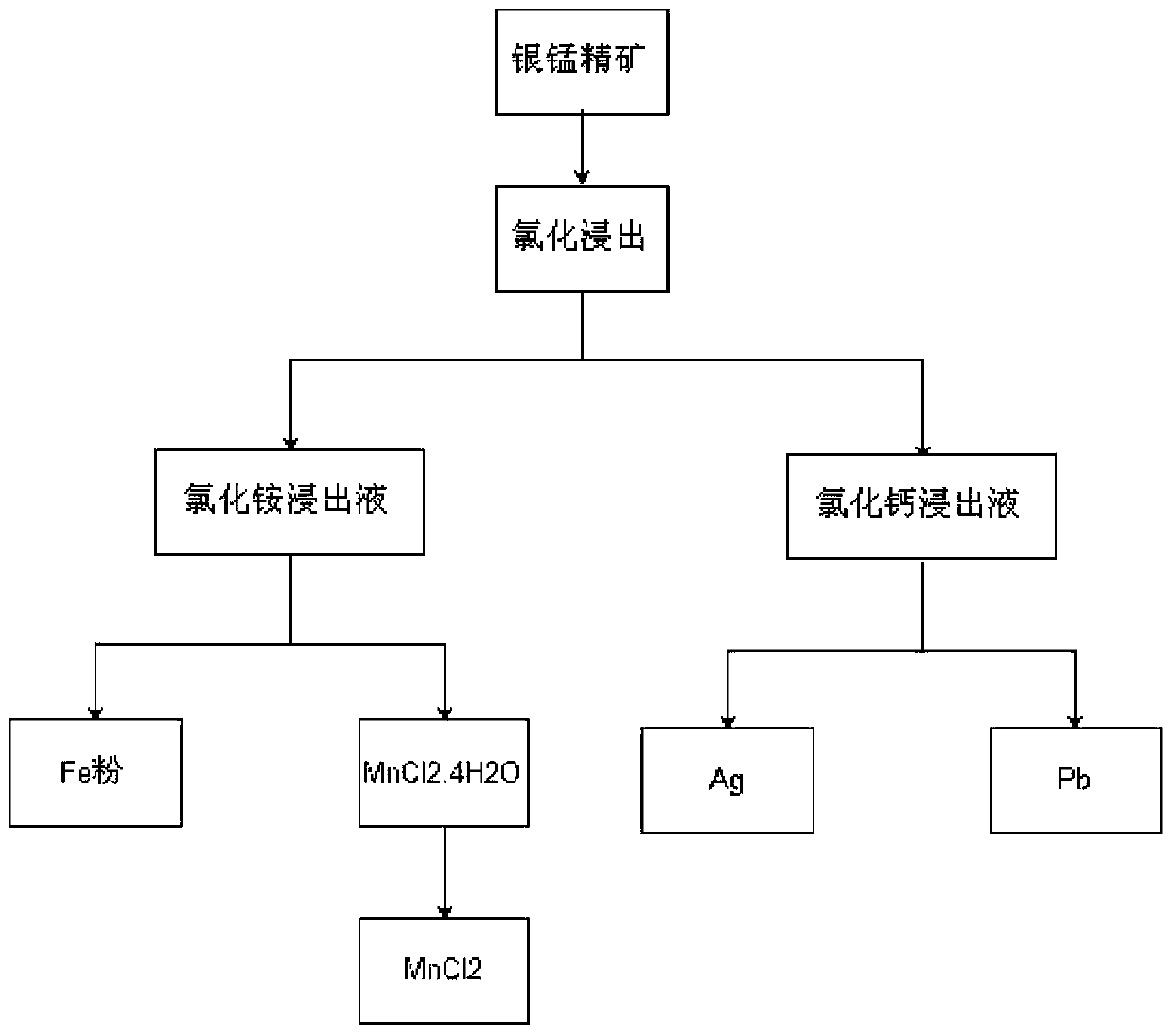
[0025] Use silver-manganese concentrate containing 190g / t of silver, 0.012g / t of gold, 20.4% of iron, 20% of manganese, and 1.37% of lead in HCl concentration of 20% by mass fraction, liquid-solid ratio of 4, and 80°C , leached for 2 hours, and the final pH value was NH 4 HCO 3 Control PH value=5±0.5, use ammonium chloride concentration to reach 150g / L and Cl - NH with a concentration of 150g / L 4 The mixed solution of Cl and HCl, the liquid-solid ratio is 4, under the condition of 80 ℃, the neutral leaching with pH=4-5 (one-time leaching) is carried out, and the leaching liquid contains Mn59-78.4g / L, Fe42-53g / L, respectively. Ag7~18mg / L, Pb0.3~0.4g / L. The leaching residue contains Ag190-220g / t and Pb2.3-3%. Mn2.5~4%, Fe3.2~4.5%, primary leaching rate (slag calculation) Mn87.5%, Fe84.3%.
Embodiment 2
[0027] With the primary chlorination leaching residue of embodiment 1 in CaCl 2Under the conditions of 300g / L, HCl mass fraction 10%, 85-90℃, and liquid-solid ratio of 5, the primary leaching of Pb and Ag was carried out for 3 hours, and the leachate contained Ag158mg / L, Pb5.8g / L, and the slag contained Ag28. 5g / t. Pb0.47%, slag-based primary leaching rate Ag85.3%, Pb79.6%.
Embodiment 3
[0029] The ammonium chloride leaching solution that contains Mn, Fe of example 1 output is at current density 200A / m 2 , tank pressure 2.0V, tank temperature 50°C. The anode plate is graphite, and the cathode plate is aluminum plate. Under the conditions of pH 3 and 5, the electrolysis is carried out for 24 hours, and the iron powder containing 82% Fe and 2.4% Mn, Mn, The separation rate of Fe reaches 97%, the cathode current efficiency is 81.2%, the solution after electrolysis contains Mn80.5g / L, Fe7g / L, and the evaporation concentration reaches above Mn130g / L to crystallize MnCl containing Mn26.7%, Fe4.2% 2 .4H 2 O (theoretical mass fraction is 32%).
[0030] Containing Ag158mg / L of example 2, the chlorinated leachate of Pb5.48g / L replaces sponge silver powder and aluminum plate replacement sponge lead with lead plate, after Pb replaces Ag, liquid contains Ag7mg / L, replacement rate 95.6%, after aluminum plate replaces Pb, liquid Containing Pb0.45g / L, the replacement rate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com