A kind of preparation method of omeprazole enteric-coated tablet
A technology of omeprazole intestine and omeprazole, which is applied in the field of drug preparation, can solve the problems of not meeting the requirements of coating, easy to induce omeprazole, large batch, etc., and achieve the effect of simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
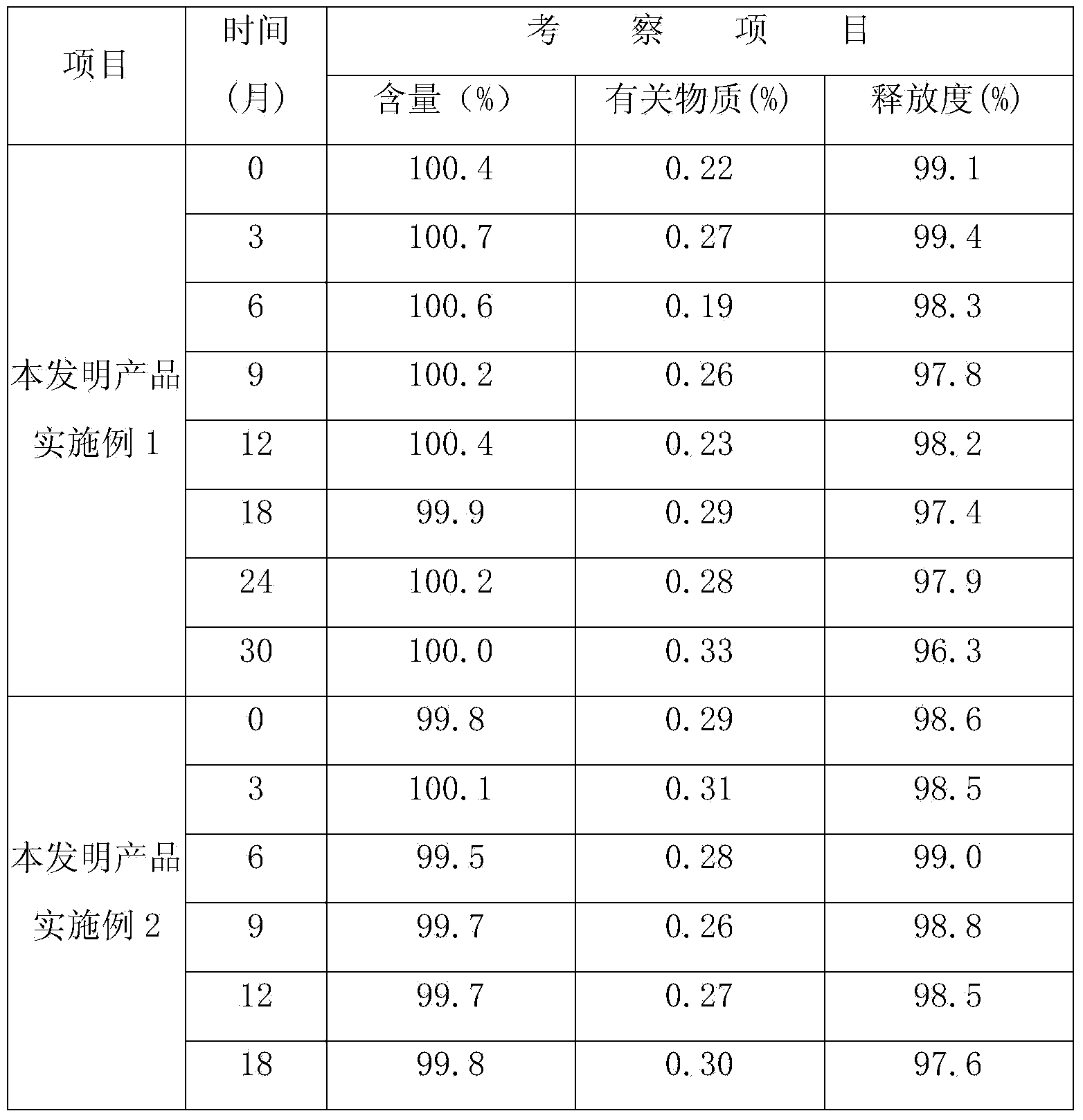
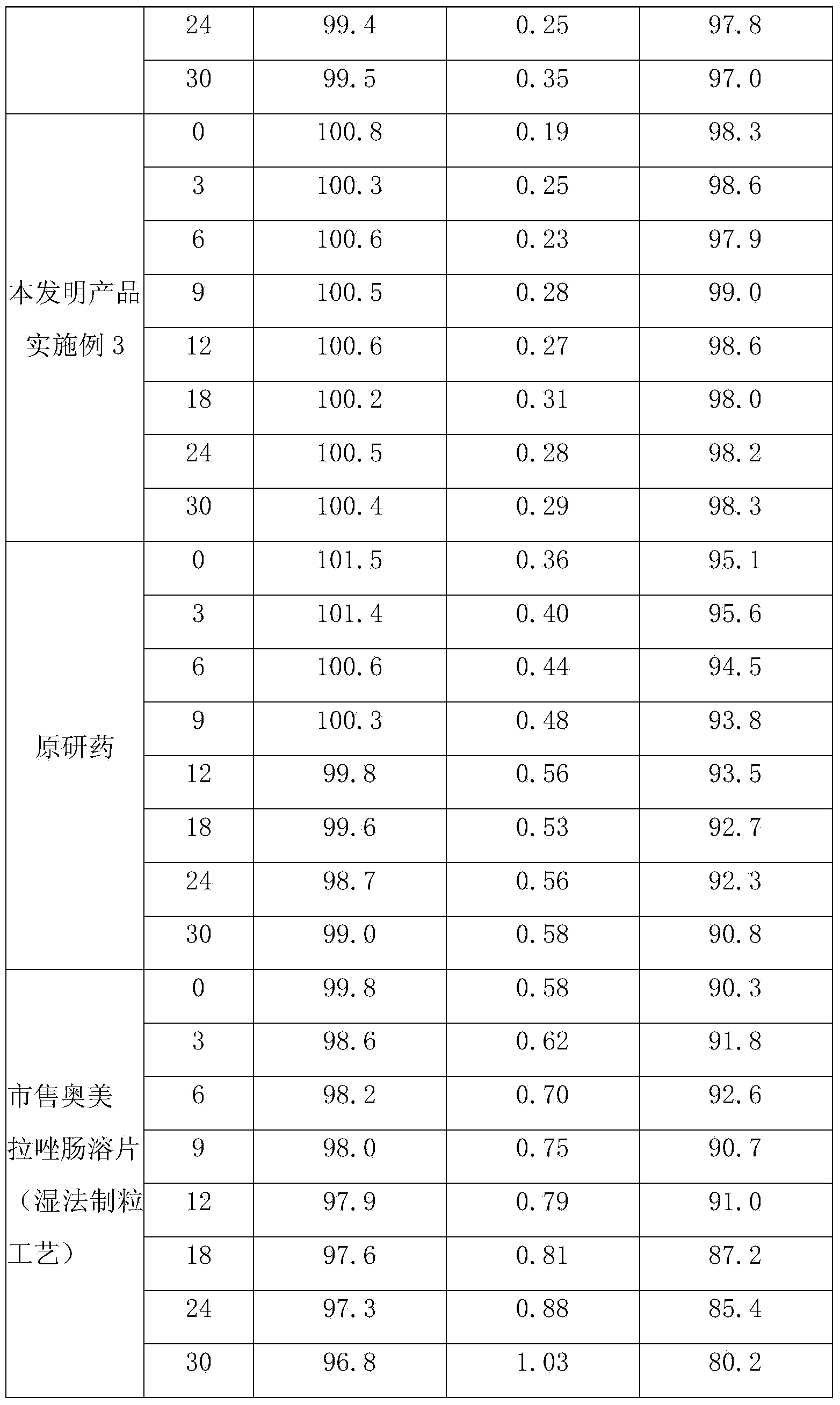
Examples
Embodiment 1
[0026] Drug specification: 10mg
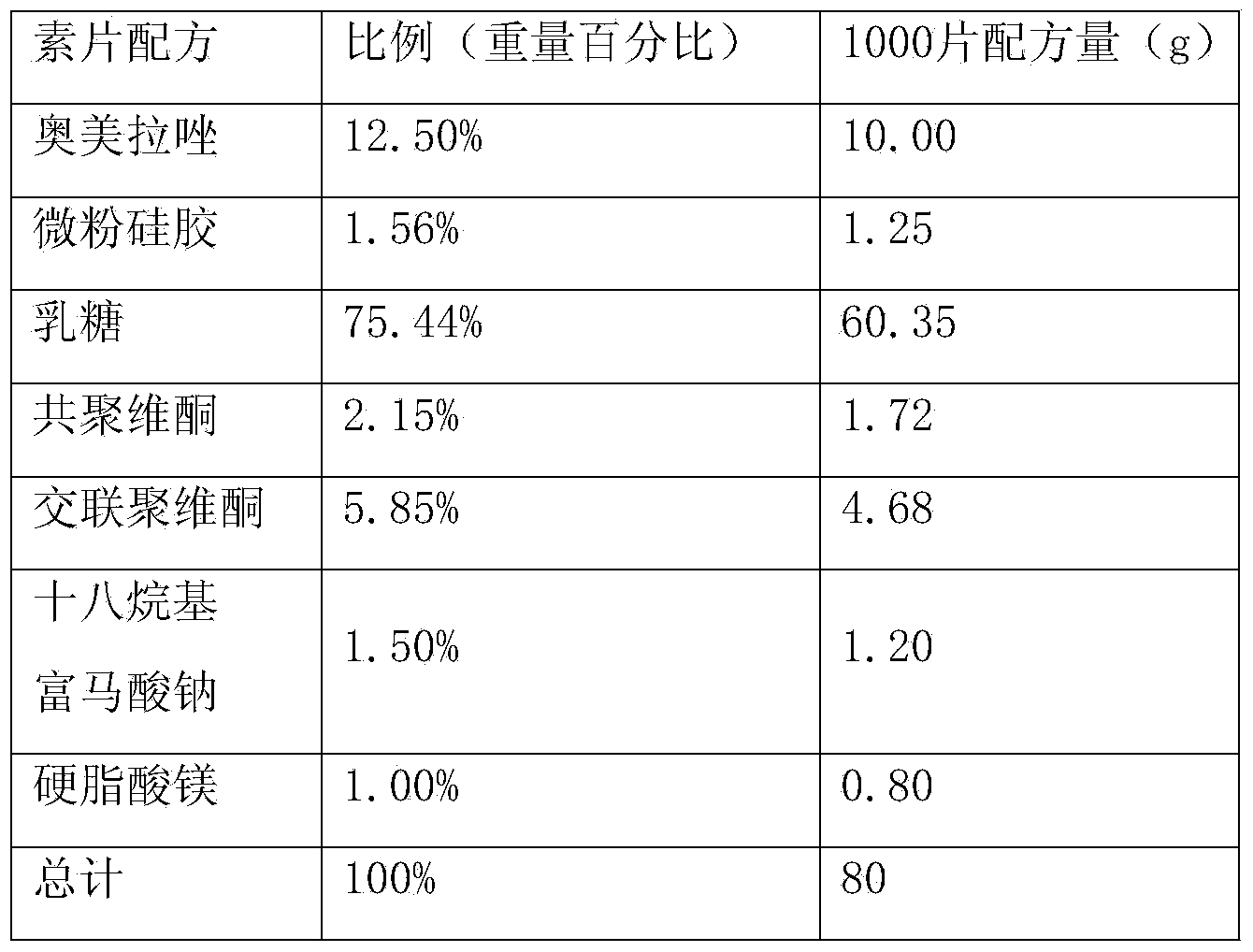
[0027] The formula is as follows:
[0028]
[0029] Preparation method: The drug formula is the same as above.
[0030] 1. Omeprazole raw material is micronized, mixed with micropowder silica gel and passed through a 200-mesh sieve; Magnesium fatty acid passed through a 100-mesh sieve, set aside;
[0031] 2. Mix the raw and auxiliary materials except magnesium stearate for 60 minutes, and mix well;
[0032]3. Put the uniformly mixed material into the dry granulator for granulation, the filling speed frequency is 10 Hz, the granulation speed frequency is 30 Hz, the granulation pressure is 2-3 MPa, the size of the granulation screen is about 30 mesh, and pass through 3-4 times. Granulate, granulate with a 60-mesh sieve to obtain an appropriate amount of dry granules;
[0033] 4. Add dry granules and magnesium stearate to the three-dimensional mixer, mix for 20 minutes until uniform, add to the hopper of the tablet press, and press into ta...
Embodiment 2
[0036] Drug specification: 20mg
[0037] The formula is as follows:
[0038] Vegetarian Tablet Formula
Ratio (weight percent)
Formula quantity for 1000 tablets (g)
25.00%
20.00
Micropowder silica gel
3.13%
2.50
60.12%
48.10
Copovidone
2.50%
2.00
Crospovidone
7.00%
5.60
Sodium Octadecyl Fumarate
1.50%
1.20
Magnesium stearate
0.75%
0.60
total
100%
80
[0039] Preparation method: The drug formula is the same as above.
[0040] 1. Omeprazole raw material is micronized, mixed with micropowder silica gel and passed through a 200-mesh sieve; Magnesium fatty acid passed through a 100-mesh sieve, set aside;
[0041] 2. Mix the raw and auxiliary materials except magnesium stearate for 60 minutes, and mix well;
[0042] 3. Put the uniformly mixed material into the dry granulator for granulation, the filling speed frequency ...
Embodiment 3
[0046] Drug specification: 40mg
[0047] The formula is as follows:
[0048] Vegetarian Tablet Formula
Ratio (weight percent)
Formula quantity for 1000 tablets (g)
Omeprazole
45.45%
40.00
Micropowder silica gel
5.68%
5.00
lactose
36.21%
31.86
Copovidone
2.05%
1.80
Crospovidone
7.95%
7.00
Sodium Octadecyl Fumarate
1.75%
1.54
0.91%
0.80
[0049] total
100%
88
[0050] Preparation method: The drug formula is the same as above.
[0051] 1. Omeprazole raw material is micronized, mixed with micropowder silica gel and passed through a 200-mesh sieve; Magnesium fatty acid passed through a 100-mesh sieve, set aside;
[0052] 2. Mix the raw and auxiliary materials except magnesium stearate for 60 minutes, and mix well;
[0053] 3. Put the uniformly mixed material into the dry granulator for granulation, the filli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com