Method for preparing aluminum chloride hexahydrate by using pulverized fuel ash as raw material through ferrous chloride induced crystallization
A technology of aluminum chloride hexahydrate and ferrous chloride, applied in the direction of aluminum chloride, aluminum halide, etc., can solve the problems of harsh operating conditions, high corrosion, complex and cumbersome process, etc., and reduce process cost and crystallization equipment requirements, the effect of improving economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
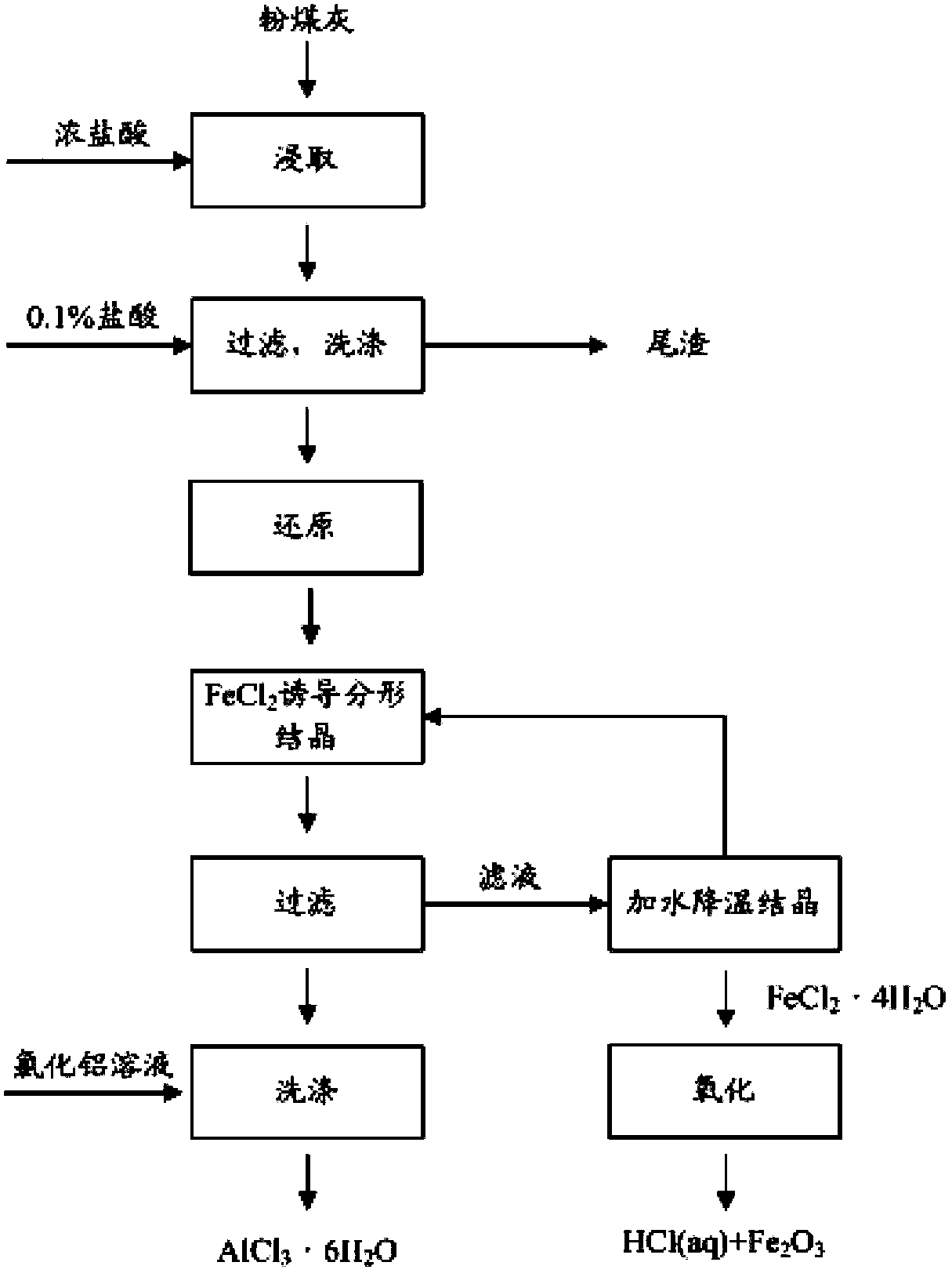
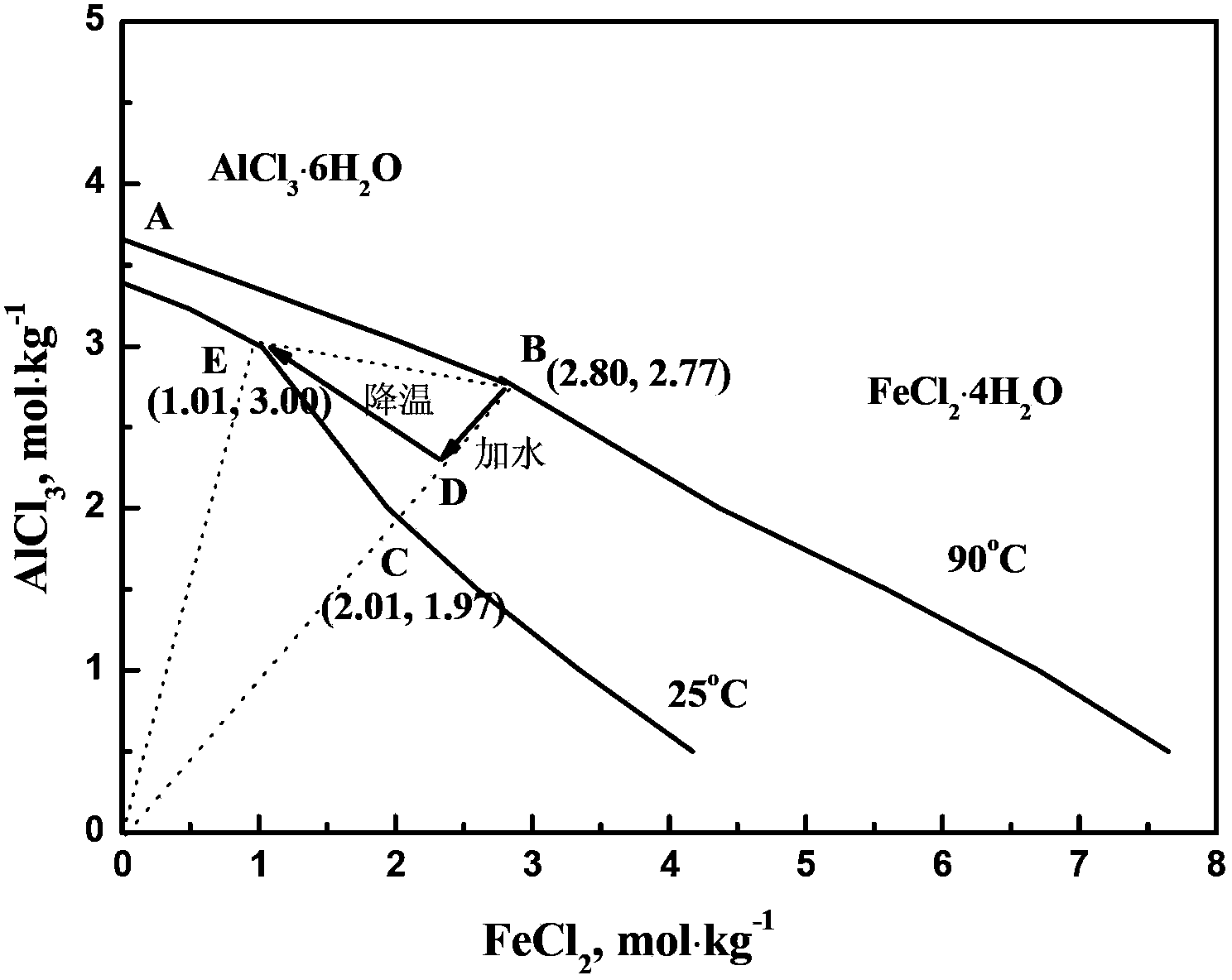
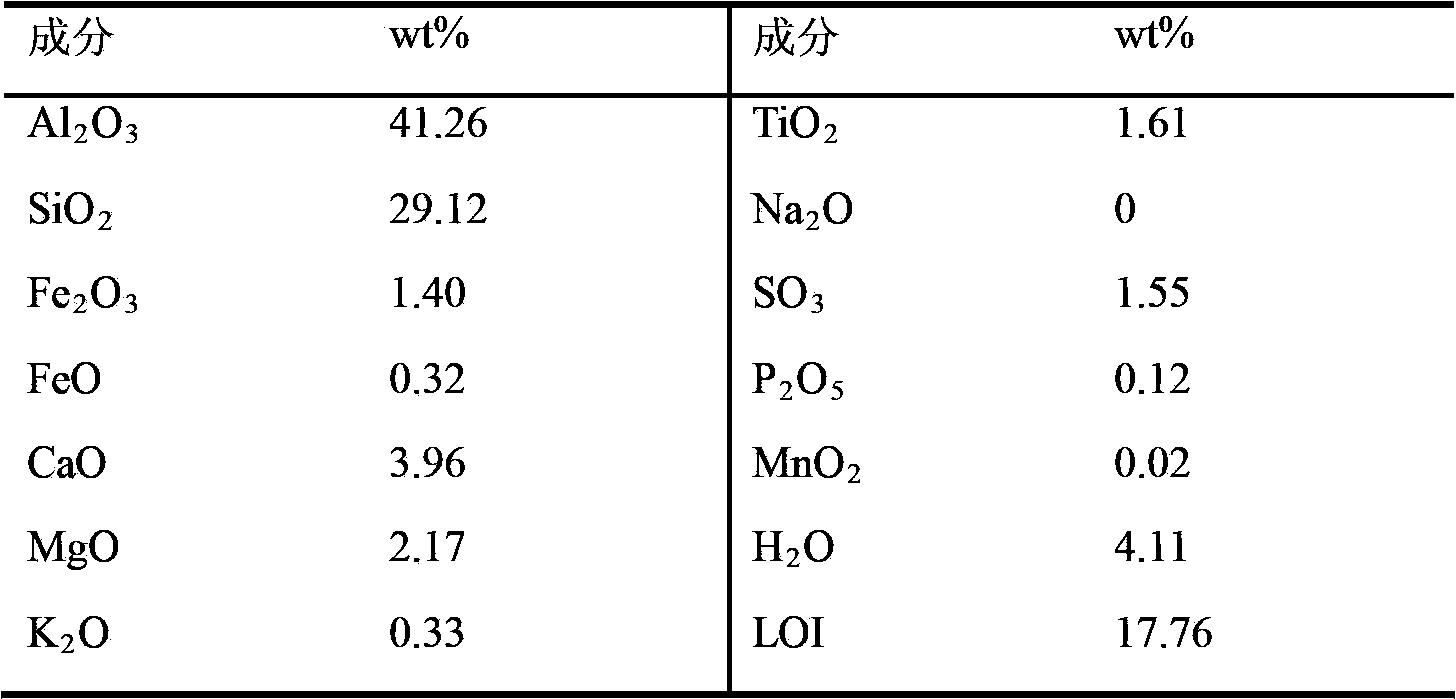
[0035] Mix 500g of certain fly ash (see Table 1 for composition) and 26% hydrochloric acid solution at a ratio of 1:1.05, place in a 4L glass reactor equipped with a condenser, and react at 80°C for 4h. After the reaction, the leaching solution is filtered, and the filtered filter residue is washed with 0.1% hydrochloric acid for more than three times, and the filter residue is collected as a raw material for making bricks for later use. Mix the filtrate with dilute hydrochloric acid washing solution and add iron filings until the electromotive force in the solution is 770mV, making Fe 3+ All reduced to Fe 2+ . Add 0.1-0.2mol of FeCl to the reduced solution 2 Carry out evaporative crystallization at 80 ℃, control the amount of evaporating water, make the precipitated crystal be aluminum chloride hexahydrate. Solid-liquid separation was carried out by filtration, and the obtained crystals were recrystallized and washed with a nearly saturated aluminum chloride solution for m...
Embodiment 2
[0041]Mix 500g of certain fly ash (see Table 1 for composition) and 28% hydrochloric acid solution at a ratio of 1:1.10, place in a 4L glass reactor equipped with a condenser, and react at 100°C for 2h. After the reaction, the leaching solution is filtered, and the filtered filter residue is washed with 0.1% hydrochloric acid for more than three times, and the filter residue is collected as a raw material for making bricks for later use. The filtrate is mixed with dilute hydrochloric acid washing solution and a small amount of iron filings are added to make the electromotive force in the solution be 770mV, so that Fe 3+ All reduced to Fe 2+ . Add 0.1-0.2mol of FeCl to the reduced solution 2 Carry out evaporative crystallization at 90°C, control the amount of evaporated water, and make the precipitated crystals be aluminum chloride hexahydrate. Solid-liquid separation was carried out by filtration, and the obtained crystals were recrystallized and washed more than three time...
Embodiment 3
[0045] Mix 500g of certain fly ash (see Table 1 for composition) and 30% hydrochloric acid solution at a ratio of 1:1.20, place in a 4L glass reactor equipped with a condenser, and react at 120°C for 1h. After the reaction, the leaching solution is filtered, and the filtered filter residue is washed with 0.1% hydrochloric acid for more than three times, and the filter residue is collected as a raw material for making bricks for later use. The filtrate is mixed with dilute hydrochloric acid washing solution and a small amount of iron filings are added to make the electromotive force in the solution be 770mV, so that Fe 3+ All reduced to Fe 2+ . Add 0.1-0.2mol of FeCl to the reduced solution 2 Carry out evaporative crystallization at 110 DEG C, control the amount of evaporating water, make the precipitated crystal be aluminum chloride hexahydrate. Solid-liquid separation was carried out by filtration, and the obtained crystals were recrystallized and washed more than three ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com