A kind of oxygen carrier for methane synthesis gas and its preparation method and application
An oxygen carrier and synthesis gas technology, applied in chemical instruments and methods, bulk chemical production, inorganic chemistry, etc., can solve the problems of oxygen carrier performance degradation, carbon black generation, complex preparation process, etc., and achieve H2 selectivity improvement, The effect of increased reactivity and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
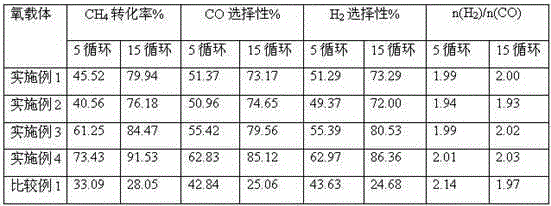
Examples
Embodiment 1
[0029] Weigh 1.18gCe(NO 3 ) 3 ﹒ 6H 2 O, 30.30gFe(NO 3 ) 3 9H 2 O, 30.83gAl(NO 3 ) 3 ﹒ 9H 2 O, put it into a 500ml beaker, slowly add distilled water until the solid is completely dissolved, and then obtain the soluble salt solution required for coprecipitation, in which the sum of Ce, Fe, and Al ion concentrations is 0.8mol / l. Prepare an ammonia solution with a concentration of 0.5 mol / l as the alkaline solution required for coprecipitation. Measure 100ml of distilled water, put it in a 1000ml beaker, and then put the beaker in a water bath at 70°C, with a stirring speed of 100r / min. Add the above-mentioned soluble salt solution and alkaline solution into the beaker drop by drop with a micro-injector, measure the pH value of the solution with pH test paper during the titration process, adjust the dropping speed of the sampler, and keep the pH of the solution between 8 and 11 . After the precipitation was complete, the dropwise addition of ammonia water was stopped,...
Embodiment 2
[0031] Weigh 2.52gCe(NO 3 ) 3 ﹒ 6H 2 O, 25.25gFe(NO 3 ) 3 9H 2 O, 29.41gAl(NO 3 ) 3 ﹒ 9H 2O, put into a 500ml beaker and slowly add distilled water until the solid is completely dissolved to obtain the required nitrate solution for co-precipitation wherein the sum of Ce, Fe, and Al ion concentrations is 0.74mol / l. Prepare a sodium hydroxide solution with a concentration of 1 mol / l as the alkaline solution required for coprecipitation. Measure 100ml of distilled water, put it in a 1000ml beaker, and then place the beaker in a water bath at 80°C, with a stirring speed of 200r / min. Add the above-mentioned soluble salt solution and alkaline solution into the beaker drop by drop with a micro-injector, measure the pH value of the solution with pH test paper during the titration process, adjust the dropping speed of the sampler, and keep the pH of the solution between 8 and 11 . After the precipitation was complete, the dropwise addition was stopped, and the stirring was ...
Embodiment example 3
[0033] Weigh 5.04gCe(NO 3 ) 3 ﹒ 6H 2 O, 13.67gFeCl 3 ·6H 2 O, 29.41gAl(NO 3 ) 3 ﹒ 9H 2 O, put it into a 500ml beaker, slowly add distilled water until the solid is completely dissolved, and then obtain the soluble salt solution required for coprecipitation, wherein the sum of Ce, Fe, and Al ion concentrations is 0.7mol / l. Prepare a potassium hydroxide solution with a concentration of 2 mol / l as the alkaline solution required for coprecipitation. Measure 100ml of distilled water, put it in a 1000ml beaker, then put the beaker in a water bath at 90°C, and the stirring speed is 300r / min. Add the above-mentioned soluble salt solution and potassium hydroxide solution drop by drop into the beaker with a micro-injector, measure the pH value of the solution with pH test paper during the titration process, adjust the dropping speed of the sampler, and keep the pH of the solution between 8 and 11 between. After the precipitation was complete, the dropwise addition was stopped...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com