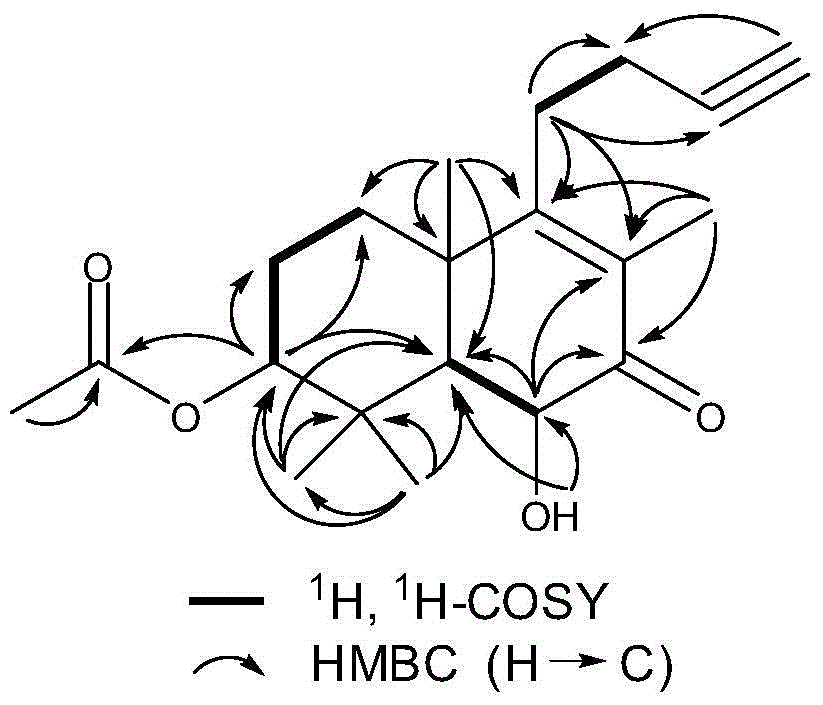Terpenoid and preparation method and application thereof
A technology for terpenoids and hydrates, which is applied in the field of terpenoids and their preparation, can solve the problems such as failure to elucidate the effective material basis of Motherwort herbal medicine, lack of research, influence on development and application, and the like, so as to shorten the time of thrombin Original time, the effect of enhancing blood coagulation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
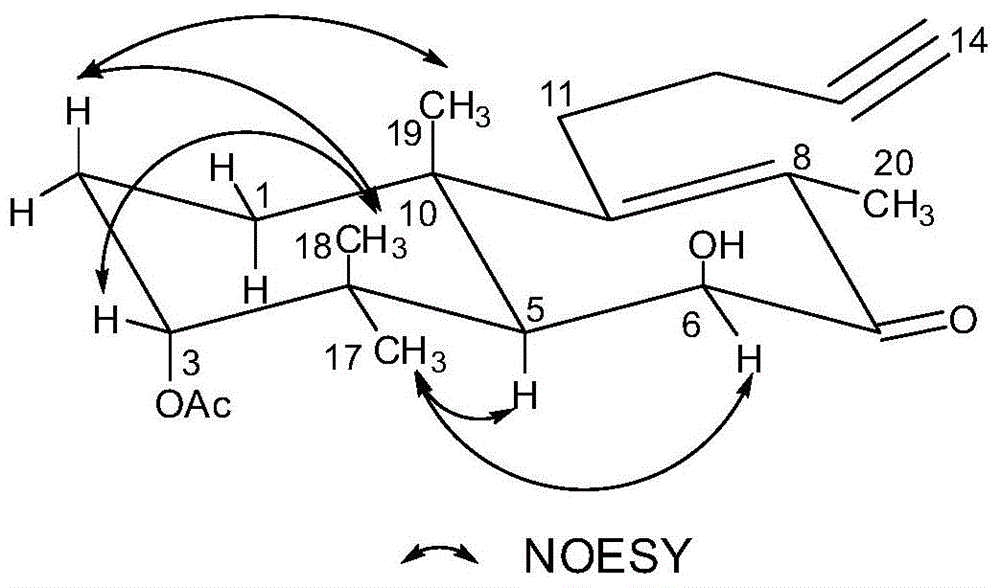
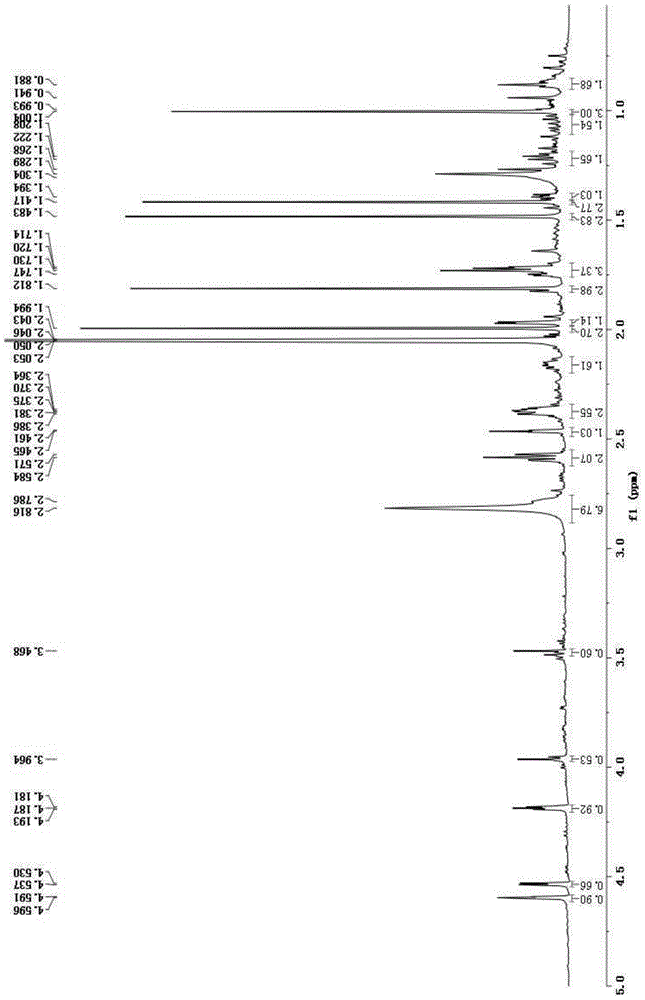
[0038] Embodiment 1 (-)-(3α, 6β)-3-acetyl-6-hydroxyl-15,16-nor-docahelane-8(9)-en-13-yn-7-ketone (formula III compound) extraction, separation and preparation
[0039] (1) Experimental materials:
[0040] ① Medicinal materials
[0041] Motherwort is collected from Wenjiang District, Chengdu, Sichuan, and identified as the whole herb of Lamiaceae Leonurus japonicus Houtt.
[0042] ②Reagents and fillers
[0043] Column chromatography silica gel, 200-300 mesh (reagent grade), purchased from Qingdao Haiyang Silica Gel Desiccant Factory;
[0044] TLC silica gel G, GF 254 and H (chemically pure), purchased from Qingdao Haiyang Silica Gel Desiccant Factory;
[0045] MCI gel CHP20P, 75-150 μm, is a reversed-phase polystyrene resin, purchased from Mitsubishi Chemical Corporation of Japan;
[0046] Sephadex LH-20 glucan gel, purchased from Sweden Amersham company;
[0047] GF 254 Silica gel preparation thin layer, purchased from Yantai Jiangyou Silica Gel Development Co., Ltd.; ...
Embodiment 2
[0066] Example 2 (-)-(3α, 6β)-3-acetyl-6-hydroxyl-15,16-nordicarbahane-8(9)-en-13-yne-7-ketone (formula III compound) extraction, separation and preparation
[0067] (1) Experimental materials are the same as in Example 1;
[0068] (2) The extraction of medicinal materials is the same as in Example 1;
[0069] (3) Separation and purification of components:
[0070] ① Disperse 1.2Kg of ethanol extract with water (10L), extract with ethyl acetate (3×10L) and n-butanol (3×10L) successively, combine the ethyl acetate part, recover the solvent under reduced pressure, and obtain ethyl acetate extract Cream 400g;
[0071] ②Use silica gel column chromatography to separate the ethyl acetate extract. The type and amount of silica gel are 200-300 mesh and 4Kg respectively, and the petroleum ether-acetone=(100:1)-(0:1) gradient elution is used to collect Petroleum ether-acetone (8:1) elution fraction, denoted as F 6 ;
[0072] ③Further use MCI resin column (reversed-phase polystyren...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com