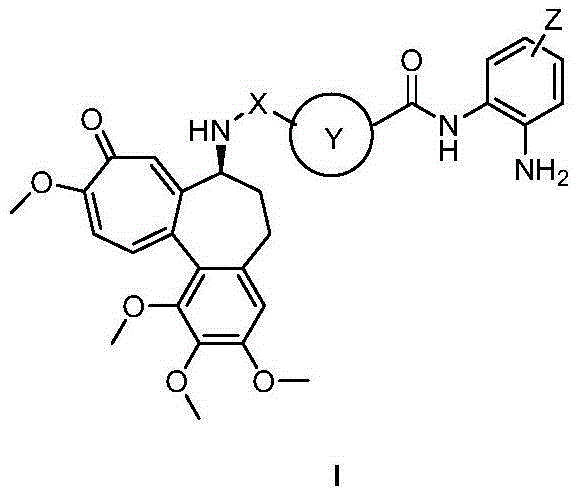
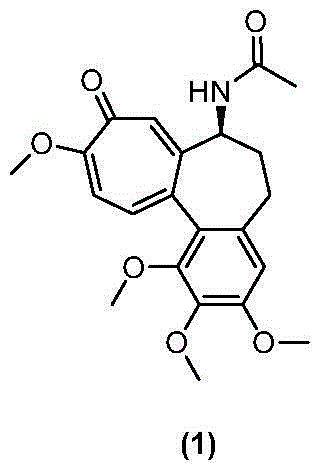
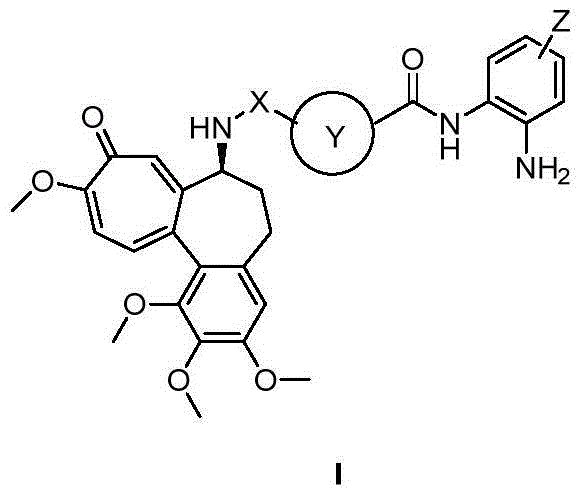
Colchicine derivative containing histone deacetylase inhibitory activity, preparation method and application thereof
A technology of sirtuin and colchicine, which is applied in the field of new colchicine derivatives, can solve the problems of complex administration methods, single target, and inconvenient clinical research, and achieve good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1.1 Preparation of compound 3
[0033] Dissolve 900 mg of monomethyl terephthalate in 10 mL of toluene, add 2.1 mL of thionyl chloride dropwise at room temperature, then raise the temperature to 60-70°C for 2 hours, remove the solvent and excess thionyl chloride under reduced pressure to obtain a colorless liquid . The liquid was dissolved in 5 mL of dichloromethane, and added dropwise to a dichloromethane solution dissolved with 1.1 mL of triethylamine and 832 mg of tert-butyl 2-aminophenylcarbamate, and reacted at room temperature for 30 minutes to stop the reaction. After extraction with dichloromethane, washing with 1N hydrochloric acid and saturated sodium carbonate, the organic phase was dried and spin-dried. The crude product was crystallized in petroleum ether-ethyl acetate to obtain 1.28 g of a white solid, with a yield of 86.5%. 1 H NMR (400MHz, DMSO) δ9.98(s,1H),8.71(s,1H),8.15–8.03(m,4H),7.55(dd,J=13.6,7.8Hz,2H),7.27–7.08( m,2H), 3.91(s,3H), 1.44(s,9H). ...
Embodiment 2
[0035] 1.2 Preparation of compound 4
[0036] At room temperature, 50 mg of compound 3 and 11 mg of lithium hydroxide monohydrate were dissolved in a mixture of 3 mL of ethanol and 0.27 mL of water. After reacting for 1 hour, 1N hydrochloric acid was added to adjust the pH to weak acidity, and the solvent was removed under reduced pressure. The resulting solid, 53 mg deacetylcolchicine, 43 mg diisopropylethylamine, 44 mg 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluoro Phosphate ester was dissolved in 5mL of dichloromethane, reacted at room temperature for two hours, extracted with dichloromethane, washed with 1N hydrochloric acid, washed with water, and the organic phase was dried, separated by column using dichloromethane-methanol as developing solvent, and 62 mg of light yellow solid was obtained , yield 81.1%. 1 H NMR (400MHz, CDCl 3 )δ9.38(s,1H),7.84–7.67(m,6H),7.63(s,1H),7.41–7.35(m,2H),7.23–7.13(m,3H),6.90(d,J= 10.9Hz,1H),6.58(s,1H),4.97–4.81(m,1H),4.00...
Embodiment 3
[0038] 1.3 Preparation of compound 5
[0039]Compound 4 was dissolved in 20 mL of dichloromethane, 3 mL of trifluoroacetic acid was added, and reacted at room temperature for 3 hours. Add saturated sodium carbonate solution to quench the reaction, extract with dichloromethane, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate and distill under reduced pressure. The obtained crude product was slurried with dichloromethane-petroleum ether to obtain 31 mg of white solid with a yield of 69.6%. 1 H NMR (400MHz, CDCl 3 )δ9.32(s,1H),8.50(d,J=6.3Hz,1H),7.78(s,1H),7.70(d,J=8.0Hz,2H),7.51(d,J=7.8Hz, 2H),7.37(d,J=10.8Hz,1H),7.26–7.23(m,1H),7.00(t,J=7.4Hz,1H),6.94(d,J=11.0Hz,1H),6.84– 6.68(m,2H),6.56(s,1H),4.98–4.83(m,1H),4.02(s,3H),3.92(s,3H),3.90(s,3H),3.55(s,3H) ,2.61–2.47(m,1H),2.42–2.25(m,2H),2.21–2.09(m,1H).HPLC:room temperature;t R =9.35min, UV 254 =95.6%;HRMS(ESI):m / z calcd for C 34 h 33 N 3 o 7 Na(M+Na + ):618.2216,found:61...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com