Synthesis method for cationic bleaching activating agents with surface activity
A bleach activator, cationic technology, applied in the field of synthesis of cationic bleach activators, can solve the problems of limited application, poor water solubility, low activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
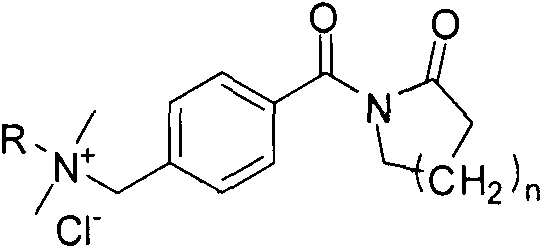
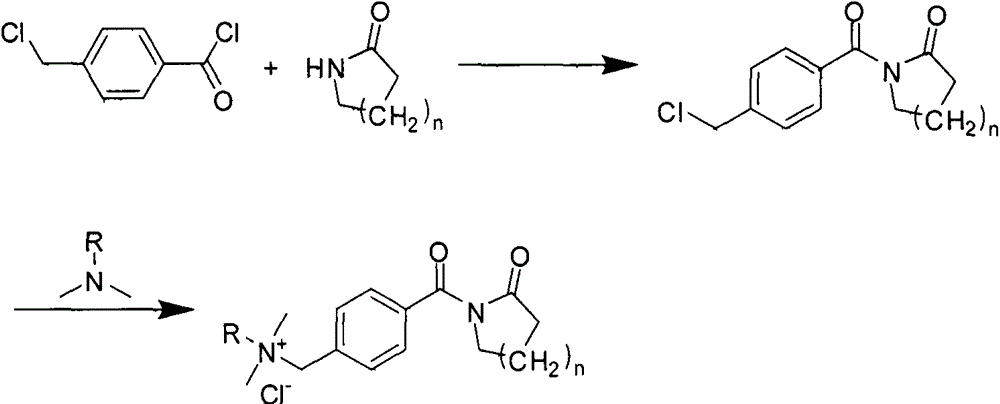
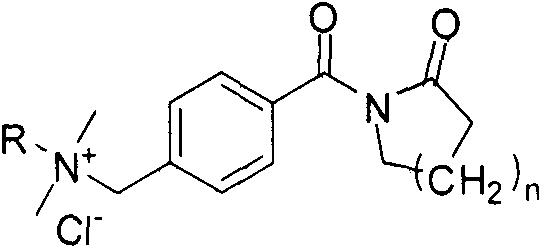
[0013] Example 1: Synthesis of 4-chloromethylbenzoyl valerolactam
[0014] Add 0.1 mol of valerolactam into a 500 mL three-neck flask filled with 0.15 mol of triethylamine and 110 mL of toluene, and after being protected by argon, slowly heat to boiling and reflux under magnetic stirring. Slowly add 50 mL of toluene solution dissolved with 0.1 mole of 4-chloromethylbenzoyl chloride into a three-necked flask, condense and reflux for 6 hours under magnetic stirring. After the reaction is complete, cool to room temperature. After filtration, the filtrate was refrigerated overnight. The precipitated white precipitate was filtered off, washed with cold toluene and dried in vacuum for use.
example 2
[0015] Example 2: N-[4-(Dimethylhexylammoniummethylene)benzoyl]caprolactam chloride
[0016] Dissolve 0.1 mole of 4-chloromethylbenzoyl valerolactam in 150 mL of acetonitrile, and after being protected by argon, slowly heat to boiling under magnetic stirring and reflux, then dissolve 50 mL of 0.15 mole of dimethylhexylamine The acetonitrile solution was added slowly and reacted for 6 hours. After the reaction was completed, the solvent was removed by evaporation. 100 mL of acetone was added at room temperature, and the mixture was rapidly heated and then cooled to room temperature. After standing for some time, the precipitated white solid was filtered off and dried.
example 3
[0017] Example 3: N-[4-(Dimethyldodecylammoniummethylene)benzoyl]caprolactam chloride
[0018] Dissolve 0.1 mole of 4-chloromethylbenzoyl valerolactam in 150 mL of tetrahydrofuran, and after being protected by argon, slowly heat to boiling under magnetic stirring and reflux, then dissolve 50 mL with 0.15 mole of dimethyl dodecane The tetrahydrofuran solution of base amine was added slowly, and reacted for 6 hours. After the reaction was completed, the solvent was removed by evaporation. 100 mL of n-hexane was added at room temperature, and the mixture was rapidly heated and then cooled to room temperature. After standing for some time, the precipitated white solid was filtered off and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com