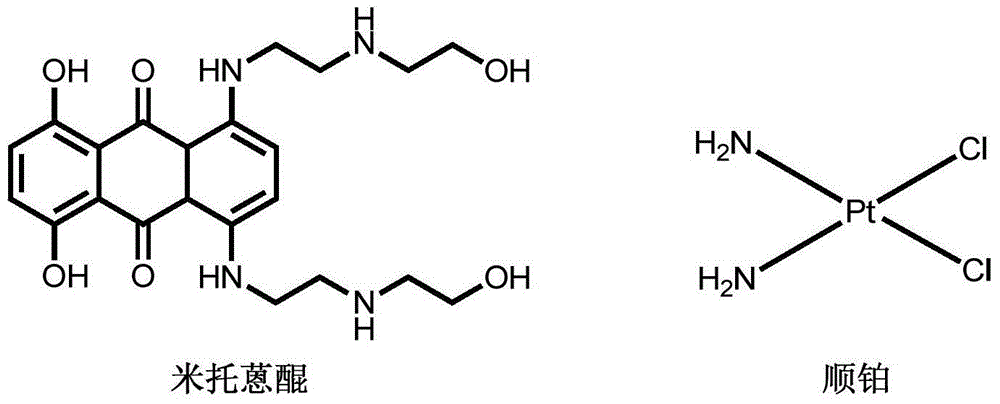
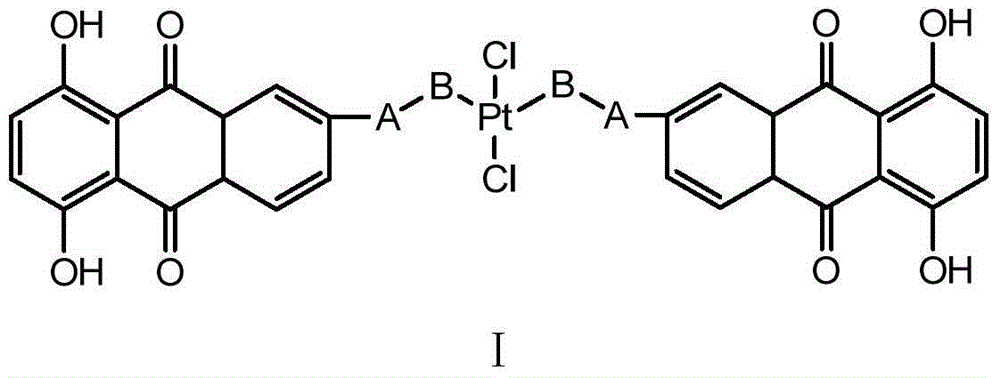
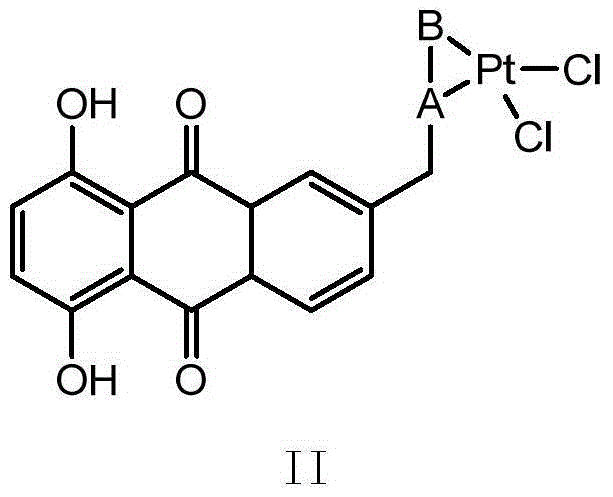
6-(azacyclo-substitute) anthraquinone platinous chloride complex as well as preparation method and application thereof
A technology of anthraquinone platinum dichloride and nitrogen heterocycle, which is applied in the field of medicine, can solve the problems of poor targeting, high toxicity, and single biological activity, and achieve good anti-tumor activity and safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Bis[6-tetrazolium-1,4-dihydroxy)-anthraquinone] platinum dichloride complex (compound 1)
[0021]
[0022] Compound 1
[0023] 1. Synthesis of 6-methyl-1,4-dihydroxyanthraquinone
[0024]
[0025] Under nitrogen protection conditions, add 5.00 g (31 mmol) 4-methylphthalic anhydride, 3.74 g (34 mmol) hydroquinone and 15.0 g (0.11 mol) aluminum trichloride. The mixture was stirred at 220°C for 2 hours, cooled to 25°C, then poured into 300 ml of ice water, acidified with 12N hydrochloric acid, the precipitated solid was filtered, washed with water, and dried in vacuo to obtain the product 6-methyl-1,4 - Dihydroxyanthraquinone 6.28 g (yield: 80%).
[0026] MS:255m / z(M+H). 1 H-NMR (DMSO-d 6 ):δ12.94(s,1H),12.90(s,1H),8.22(d,J=8Hz,1H),8.12(s,1H),7.63(d,J=8Hz,1H),7.29(s ,2H),2.55(s,3H);
[0027] 2. Synthesis of 6-methyl-1,4-bis(methoxymethyl ether)-anthraquinone
[0028]
[0029] Under nitrogen protection conditions at 0°C, 6-methyl-1,4-dihydroxya...
Embodiment 2
[0052] Example 2: Bis-6-[(3-aminopyridine)methyl]-1,4-dihydroxyanthraquinone platinum dichloride complex (compound 2)
[0053]
[0054] Compound 2
[0055] 1. Synthesis of 6-[(3-aminopyridine)methyl]-1,4-bis(methoxymethyl ether)anthraquinone
[0056]
[0057] Under nitrogen protection, 5.06 g (12 mmol) of 6-bromomethyl-1,4-bis(methoxymethyl ether)-anthraquinone, 1.32 g (12 mmol) of 3-aminopyridine were added to a 500 ml eggplant-shaped bottle. mol), 3.31 grams of potassium carbonate (24 mmol), 300 milliliters of acetone solvent, after reflux and stirring for 4 hours, the solution was spin-dried, extracted with 100 milliliters of ethyl acetate and 100 milliliters of water, separated the ethyl acetate phase and then subtracted Concentrated under reduced pressure, and the crude product was separated by column to obtain 4.35 g of the product. (83% yield).
[0058] MS:435m / z(M+H).
[0059] 1 H-NMR (DMSO-d 6 ):δ8.28(d,J=8.2Hz,1H),8.21(s,1H),7.81(dd,J=1.2,J=4.0Hz,1H),7....
Embodiment 3
[0065] Example 3: Bis-6-[(1-methyl-5-mercapto)methyl]-1,4-dihydroxyanthraquinone platinum dichloride complex (compound 3)
[0066]
[0067] Compound 3
[0068] 1. Synthesis of 6-[(1-methyl-5-mercapto)methyl]-1,4-bis(methoxymethyl ether)anthraquinone
[0069]
[0070] Under nitrogen protection, add 5.06 g (12 mmol) of 6-bromomethyl-1,4-bis(methoxymethyl ether)-anthraquinone, 1-methyl-5-mercapto-tetra 1.74 g (15 mmol) of oxazole, 0.6 g of sodium hydroxide (15 mmol), 100 ml of N,N-dimethylformamide solvent, stirred at 60°C for 3 hours, then mixed with 100 ml of ethyl acetate, 100 Extract with milliliter of water, separate the ethyl acetate phase and concentrate under reduced pressure. The crude product is recrystallized from hot acetone to obtain 5.02 g of the product. (Yield 91%).
[0071] MS:457m / z(M+H).
[0072] 1 H-NMR (DMSO-d 6 ):δ8.16(d,J=8.0Hz,1H),8.10(s,1H),7.77(d,J=8.2Hz,1H),7.42(s,2H),6.24(s,4H),4.15 (s,2H),3.57(s,6H),2.63(s,3H);
[0073] 2. Synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com