Cefathiamidine monocrystal
A technology of cefathiamidine and single crystal, which is applied in the direction of single crystal growth, single crystal growth, crystal growth, etc., and achieves the effects of good stability, simple equipment, and easy preparation and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
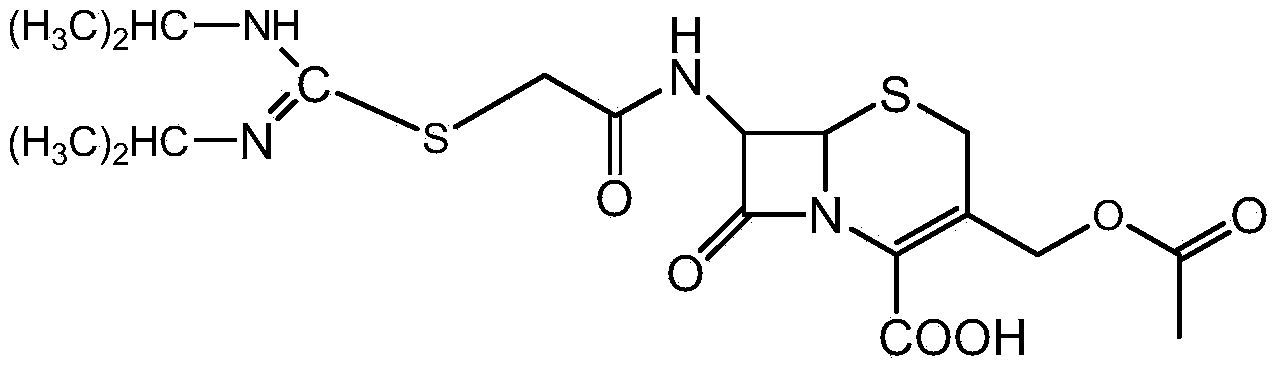
[0039] Add 12 g of cefathiamidine into a clean Erlenmeyer flask, then add 9.4 ml of water and 47.6 ml of ethanol in order, and dissolve at room temperature. Then, 37.3 ml of ethanol and 5.7 ml of acetone were added. Finally, a 0.12 g / ml cefathiamidine solution [equivalent to 100 ml of a mixed solvent of ethanol / water / acetone (volume ratio 9:1:0.6)] was prepared. Seal it with plastic film, and punch some small holes in the plastic film. Store at a certain temperature of 2-6°C. Stand still for 96 hours, that is.
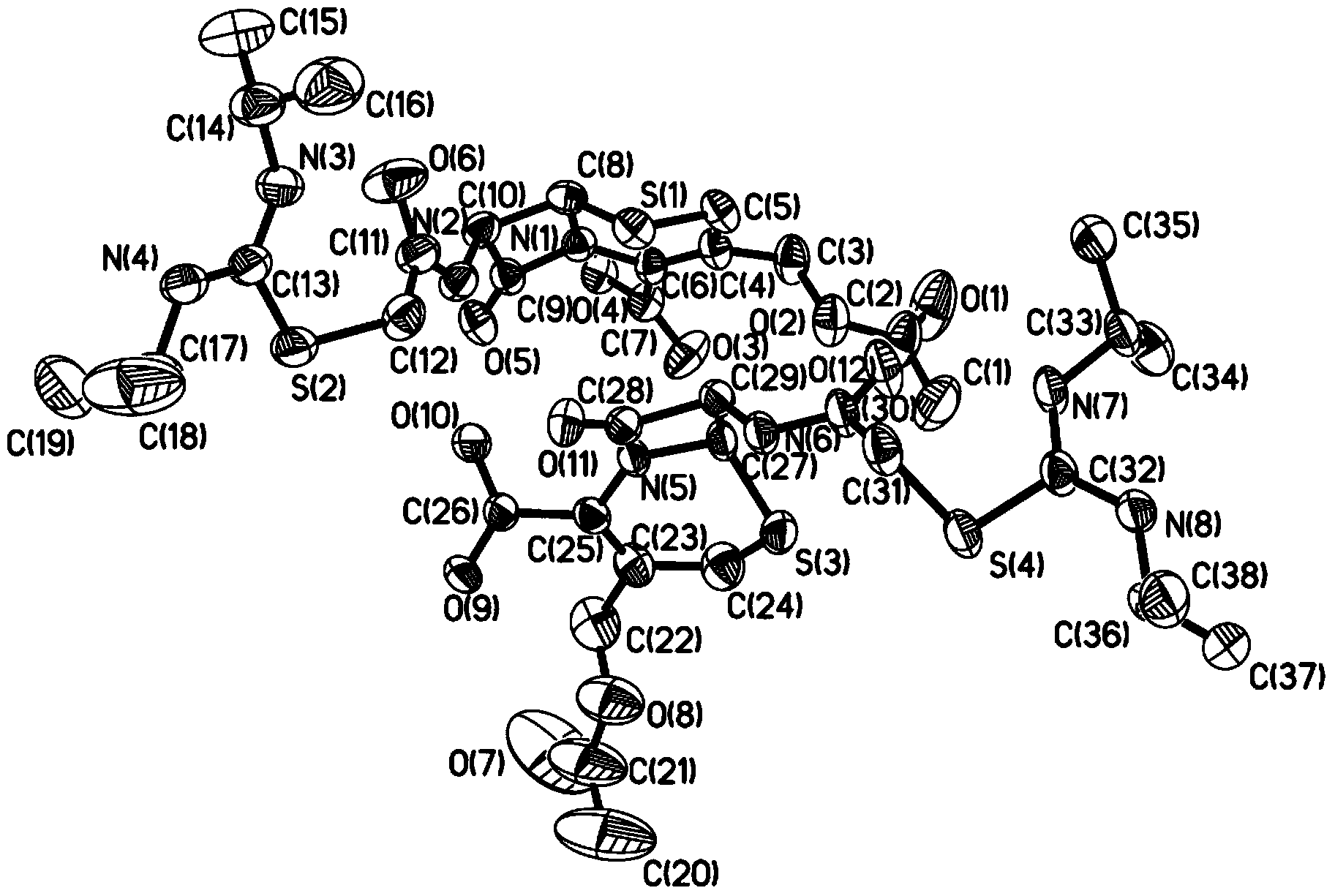
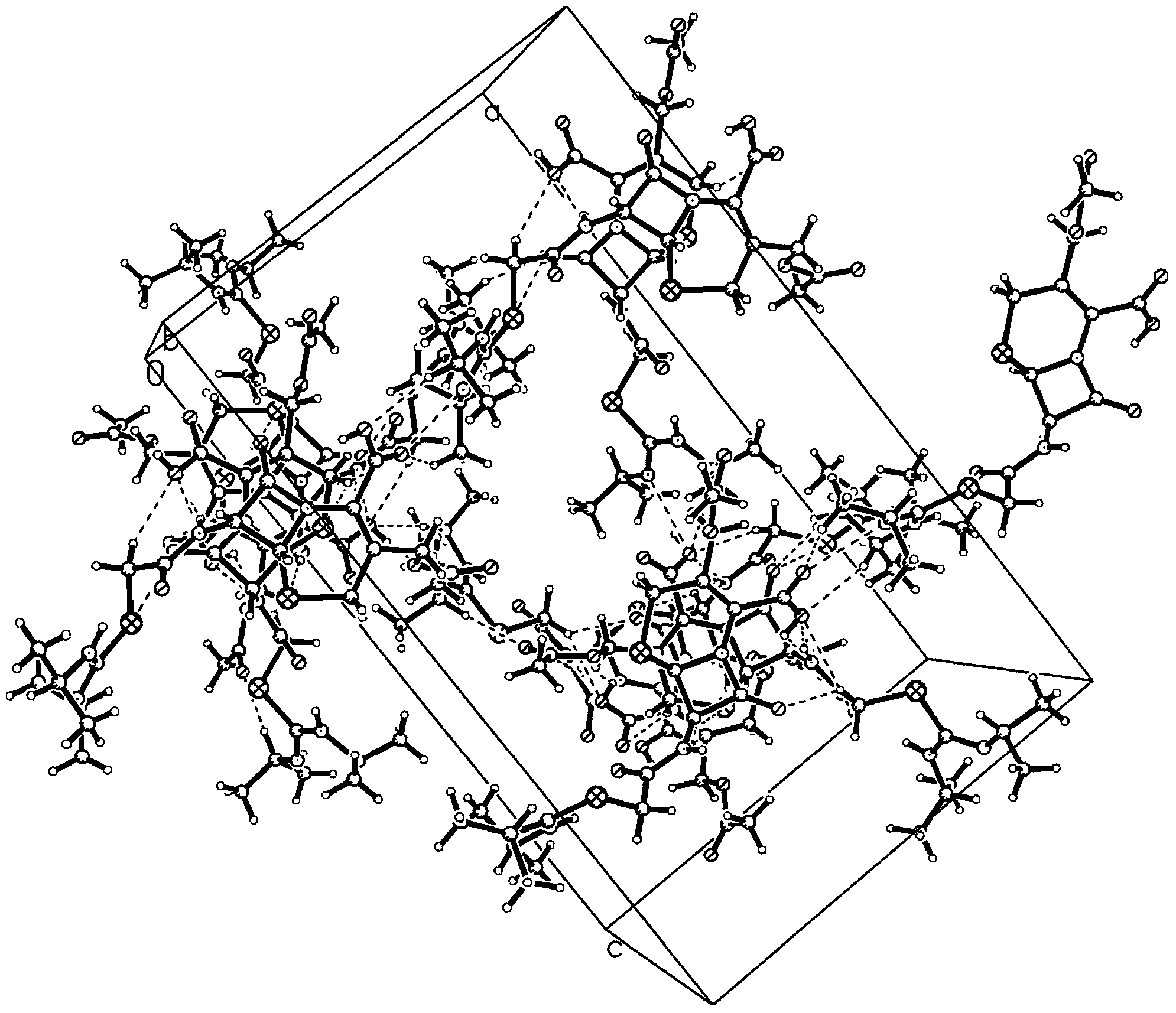
[0040] Adopt R-AXIS-RAPID X-ray single crystal diffractometer of Japan Rigaku Company to analyze the obtained crystal sample, the results are shown in Tables 1-5 and attached Figure 1~2 ,in:
[0041] Table 1 is crystal data and structure optimization results;
[0042] Table 2 is the coordinates of non-hydrogen atoms
[0043] Table 3 key length
[0044] Table 4 bond angle (°);
[0045] Table 5 hydrogen atom coordinates
[0046] figure 1 is the molecular...
Embodiment 2
[0050] Add 100ml of a mixed solvent of ethanol / water / acetone (4:1:0.05 by volume) into a clean Erlenmeyer flask, then add 5g of cefathiamidine to prepare a 0.15g / ml cefathiamidine solution. Seal it with plastic film, and punch some small holes in the plastic film. Store at a certain temperature of 0-8°C. Stand still for 96 hours, that is.
[0051] The obtained cefathiamidine crystal diffraction experiment is shown in attached table 1~5 and attached table Figure 1~2 .
Embodiment 3
[0053] Add 75ml of ethanol / water mixed solvent with a volume ratio of 10:1 in a clean Erlenmeyer flask, then add 50g of cefathiamidine, dissolve at room temperature, then add dropwise 25ml of acetone to prepare a 0.5g / ml cefathiamidine solution【 Equivalent to 100ml of a mixed solvent with a volume ratio of ethanol / water / acetone of 10:1:3.67]. Seal it with plastic film, and punch some small holes in the plastic film. Store at a certain temperature of 7-15°C. Stand still for 96 hours, that is.
[0054] The obtained cefathiamidine crystal diffraction experiment is shown in attached table 1~5 and attached table Figure 1~2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com