System and microorganism for producing taxadiene, and application of system or microorganism
A technology of taxadiene and microorganisms, applied in the field of synthetic biology, to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Mevalonate pathway plasmid construction
[0048] Escherichia coli BL21(DE3) genomic DNA and Saccharomyces cerevisiae INVSC1 genomic DNA were purified with Qiagen's Blood and Cell Culture DNA Mini Kit.
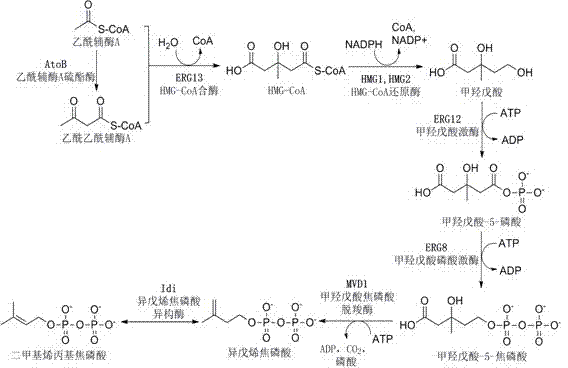
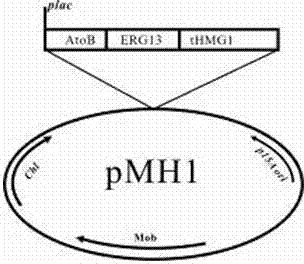
[0049] Plasmid pMH1 contains the first three genes of the mevalonate pathway: atoB gene (acetoacetyl-CoA thioesterase) from Escherichia coli BL21 (DE3), erg13 (HMG-CoA synthase, 3- hydroxymethyl-glutaryl-CoA synthase) and thmg1 (HMG-CoA reductase, 3-hydroxymethyl-glutaryl-CoA reductase, deletes the transmembrane region of HMG1). Plasmid pFZ81 contains the last four genes of the mevalonate pathway: erg12 (mevalonate kinase), erg8 (mevalonate-5-phosphate kinase) and mvd1 (mevalonate-5-phosphate kinase) from Saccharomyces cerevisiae INVSC1 pyrophosphate kinase), derived from the idi (isopentenyl pyrophosphate isomerase) gene of Escherichia coli BL21 (DE3). All genes were amplified by PCR, and the primers used are listed in Table 1.
[0050] The specific cons...
Embodiment 2
[0056] Example 2 Taxadiene Production Metabolic Pathway Plasmid Construction
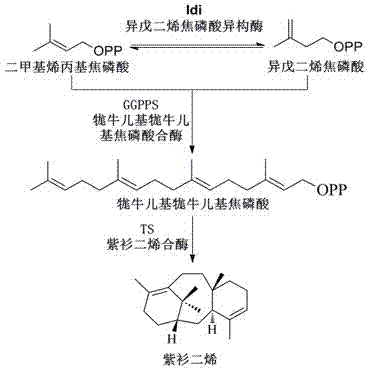
[0057] The codon-optimized geranylgeranyl pyrophosphate synthase ggpps gene derived from Taxus canadensis (sequence shown in SEQ ID NO.1) and the codon-optimized taxadiene derived from Taxus brevifolia were synthesized Enzyme ts gene for gene synthesis (sequence shown in SEQ ID NO.2), followed by primers GS-duet-f, GS-duet-r and TS-duet-f, TS-duet-r amplification, while the vector pETduet-1 was amplified with duet-TG-f, duet-TG-r, and the three fragments were connected together by Gibson method to obtain plasmid pFZ131 ( Figure 5 ). The replicon of plasmid pFZ131 was replaced with pSC01 replicon by simple cloning method, the specific operation is as follows: Plasmid pFZ131 was amplified with primers duet-pSC101-f, duet-pSC101-r, and pSC101-f, pSC101-r were used as primers for pSC101 plasmid The template amplifies the pSC101 replicon, and then connects the two fragments to obtain the plasmid pXC02...
Embodiment 3
[0058] Example 3 Construction of Taxadiene Production Strains
[0059] The two plasmids pMH1 and pFZ81 of the mevalonate pathway were simultaneously transformed into Escherichia coli BL21(DE3) to obtain BL21(DE3) / pMH1 / pFZ81, and then pXC02 was transformed into BL21(DE3) / pMH1 / pFZ81 to obtain purple The taxadiene producing strain T2. Transform pFZ131 into BL21(DE3) / pMH1 / pFZ81 to obtain taxadiene producing strain T4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com