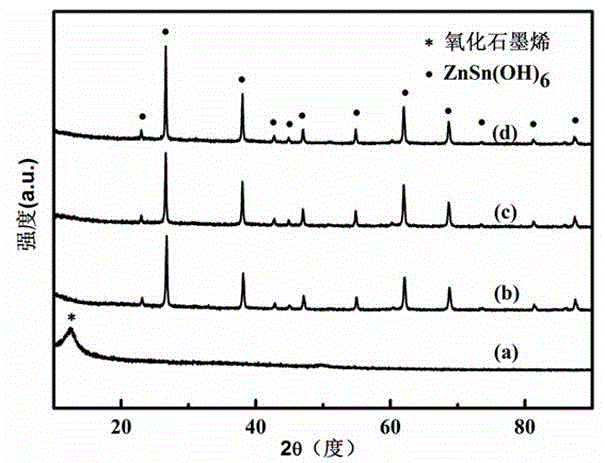
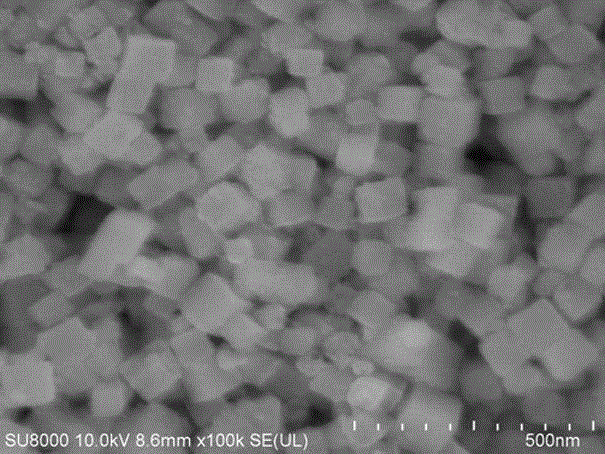
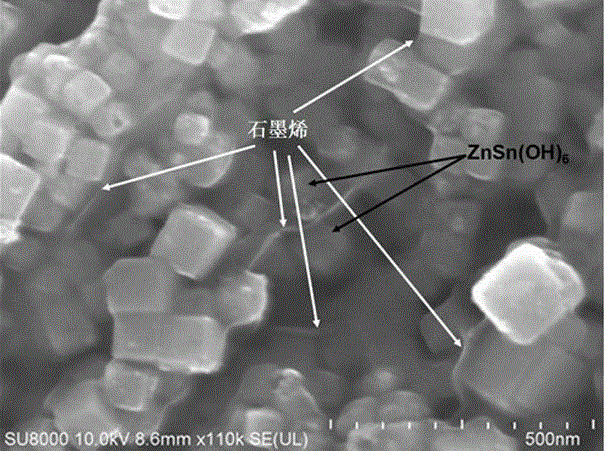
ZnSn (OH)6 nanometer cubic particle/graphene sandwich structure compound light catalyst
A nano-cubic and ene sandwich technology, applied in the field of photocatalysis, can solve the problems of difficult to control product morphology, troublesome post-processing, low product purity, etc., and achieve excellent photocatalytic effect, low production cost, and easy separation and recovery. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] preparation:
[0040] Step 1: Preparation of ZnSn(OH) 6 Nano Cubic Particles:
[0041] (1) Preparation of reaction precursor solution: Dissolve 2 mmol of zinc acetate dihydrate in 80 mL of deionized water at room temperature, then add an equimolar amount of potassium stannate to the above-mentioned zinc acetate solution, and stir evenly to form a reaction precursor solution;
[0042] (2) Hydrothermal reaction: Transfer the obtained precursor solution to a 100mL polytetrafluoroethylene reactor, raise the temperature to 180°C, and keep it warm for 18h;
[0043] (3) Washing and collection of precipitates: the obtained product is naturally cooled to room temperature, washed three times with hydrochloric acid with pH = 2.5, deionized water, and absolute ethanol, centrifuged to collect the precipitate, and vacuum-dried at 60°C to obtain ZnSn(OH) 6 Nanocubic particles.
[0044] The second step: prepare graphene oxide colloidal solution:
[0045] Using natural graphite powd...
Embodiment 2
[0053] Step 1: Preparation of ZnSn(OH) 6 Nano Cubic Particles:
[0054] (1) Preparation of reaction precursor solution: Dissolve 2 mmol of zinc acetate dihydrate in 80 mL of deionized water at room temperature, then add an equimolar amount of potassium stannate to the above-mentioned zinc acetate solution, and stir evenly to form a reaction precursor solution;
[0055] (2) Hydrothermal reaction: Transfer the obtained precursor solution to a 100mL polytetrafluoroethylene reactor, raise the temperature to 180°C, and keep it warm for 18h;
[0056] (3) Washing and collection of precipitates: the obtained product is naturally cooled to room temperature, washed three times with hydrochloric acid with pH = 2.5, deionized water, and absolute ethanol, centrifuged to collect the precipitate, and vacuum-dried at 60°C to obtain ZnSn(OH) 6 Nanocubic particles.
[0057] The second step: prepare graphene oxide colloidal solution:
[0058] Using natural graphite powder as raw material, gra...
Embodiment 3
[0064] Step 1: Preparation of ZnSn(OH)6 nano-cubic particles:
[0065] (1) Preparation of reaction precursor solution: Dissolve 2 mmol of zinc acetate dihydrate in 80 mL of deionized water at room temperature, then add an equimolar amount of potassium stannate to the above-mentioned zinc acetate solution, and stir evenly to form a reaction precursor solution;
[0066] (2) Hydrothermal reaction: transfer the obtained precursor solution into a polytetrafluoroethylene reactor with a capacity of 100 mL, raise the temperature to 180° C., and keep the temperature for 18 hours.
[0067] (3) Washing and collection of precipitates: the obtained product is naturally cooled to room temperature, washed three times with hydrochloric acid with pH = 2.5, deionized water, and absolute ethanol, centrifuged to collect the precipitate, and vacuum-dried at 60°C to obtain ZnSn(OH) 6 Nanocubic particles.
[0068] The second step: prepare graphene oxide colloidal solution:
[0069] Using natural g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com