Propranolol enantiomer resolution method
A technology of propranolol and enantiomers, which is applied in the field of pharmaceutical preparation, can solve problems such as unsatisfactory chiral extraction of enantiomers, and achieve the effects of low toxicity, low price and easy availability of raw materials in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
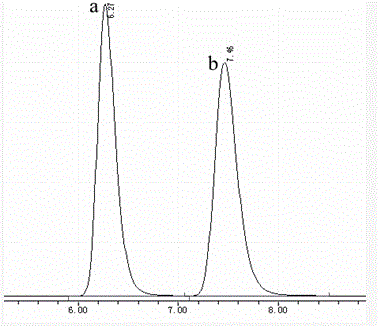
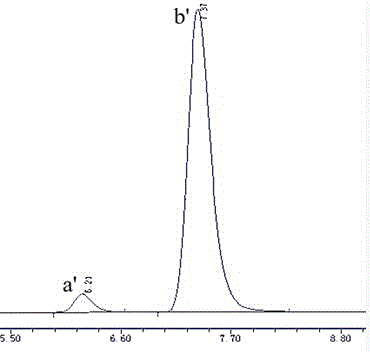
[0022] Take by weighing 100g (R, S)- propanol hydrochloride bulk drug and add 2500ml water to dissolve, add 2 times of equivalent 20% sodium hydroxide solution and carry out neutralization reaction, static, filter, wash, dry to obtain (R, S) - Propranol; the resolving agent dehydroabietic acid and the above-mentioned propranolol prepared according to the molar ratio of 1:1 feed intake, with methanol as a solvent, reacted for 1h at 30 ° C, after the reaction was completed - Cool down and crystallize at 5°C for 5 hours, filter, wash, and dry to obtain propranolol dehydroabietate; add 1500ml of methanol to the above-prepared propranolol dehydroabietate to fully dissolve, then add 3 Double the equivalent of 20% sodium hydroxide solution to react for 30 minutes, dry to obtain the crude product of S(-)-propranolol, add it to the crude product of S(-)-propranolol, fully dissolve in 2500ml of water, filter, wash and dry 41.3 g of S(-)-propranolol was obtained, and the product purity w...
Embodiment 2
[0024] Take by weighing 100g (R, S)- propanol hydrochloride crude drug and add 2500ml water to dissolve, add 3 times of equivalent 35% sodium hydroxide solution and carry out neutralization reaction, static, filter, wash, dry to obtain (R, S) -propranol; the resolving agent dehydroabietic acid and the above-mentioned propranolol prepared according to the molar ratio are 1.3:1 feeding intake, with methanol as a solvent, reacted for 3h at 25°C, after the reaction finished at 5 Cool down and crystallize at ℃ for 6 hours, filter, wash, and dry to obtain propranolol dehydroabietate; add 1500ml methanol to the above-prepared propranolol dehydroabietate to fully dissolve, add 3 times Equivalent 35% sodium hydroxide solution was reacted for 20min, dried to obtain the crude product of S(-)-propranolol, added to the crude product of S(-)-propranolol, fully dissolved in 2500ml water, filtered, washed and dried to prepare Obtain the S(-)-propranolol of 37.1g, product purity is 90.0%.
Embodiment 3
[0026] Take by weighing 100g (R, S)- propranolol hydrochloride crude drug and add 2500ml water to dissolve, add 1 times of equivalent 10% sodium hydroxide solution and carry out neutralization reaction, stand still, filter, wash, dry to obtain (R, S) -propranol; the resolving agent dehydroabietic acid and the above-mentioned propranolol prepared are fed according to the molar ratio of 1.3:1, and methanol is used as a solvent to react for 2h at 35°C. Cool down and crystallize at ℃ for 6 hours, filter, wash, and dry to obtain propranolol dehydroabietate; add 2000ml methanol to the above-prepared propranolol dehydroabietate to fully dissolve, add 3 times Equivalent 10% sodium hydroxide solution was reacted for 45min, dried to obtain the crude product of S(-)-propranolol, added to the crude product of S(-)-propranolol, fully dissolved in 2500ml water, filtered, washed and dried to prepare Obtain the S(-)-propranolol of 23.2g, product purity is 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com