Novel preparation method of 5-aminomethyl nicotinic acid
A technology of aminomethylnicotinic acid and methylnicotinic acid is applied in the field of preparation of 5-aminomethylnicotinic acid, which can solve the problems of hidden danger of explosion, high corrosiveness, intense reaction and the like, and achieves safe operation and high yield , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
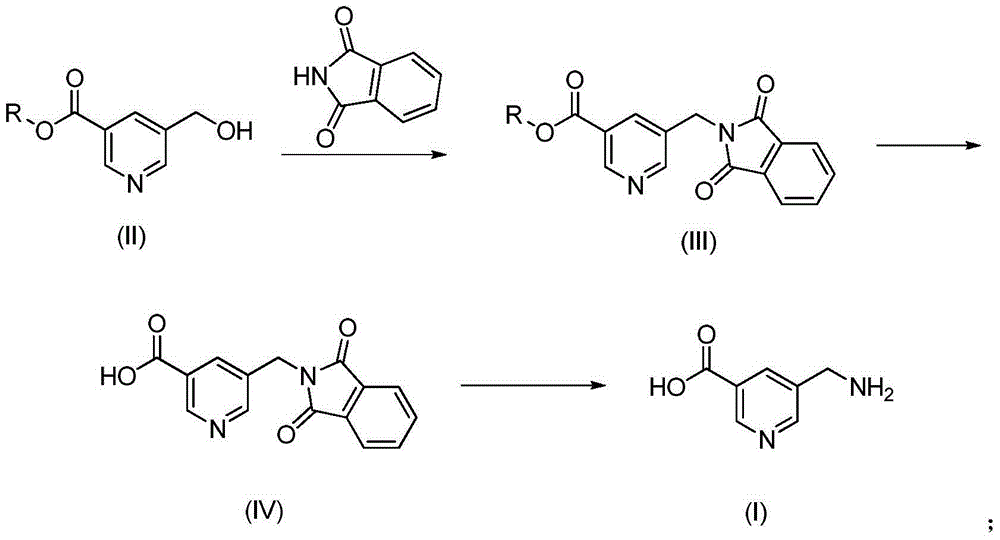
[0023] The preparation of embodiment 15-aminomethyl nicotinic acid
[0024] The preparation of 5-aminomethyl nicotinic acid comprises the following steps:
[0025] (1) Preparation of methyl 5-(phthalamido)methylnicotinate
[0026] Add 1.00g of methyl 5-hydroxymethylnicotinate, 1.56g of triphenylphosphine and 0.88g of phthalamide into a clean and dry three-necked flask, and dissolve them in 6ml of tetrahydrofuran. Add 1.11g of diethyl azodicarboxylate in tetrahydrofuran (8mL) dropwise, stir at room temperature for 12 hours, and complete reaction of methyl 5-hydroxymethylnicotinate to obtain methyl 5-(phthalamido)methylnicotinate .
[0027] (2) Preparation of 5-(phthalamido)methylnicotinic acid
[0028] 5-(phthalamido) methyl nicotinic acid aqueous solution, slowly dropwise add 1.0 M sodium hydroxide aqueous solution to adjust the pH of the reaction system to 14, stir for 1 h, and the hydrolysis is complete by HPLC detection. Ethyl acetate was added for extraction. Concentr...
Embodiment 25
[0031] The preparation of embodiment 25-aminomethyl nicotinic acid
[0032] The preparation of 5-aminomethyl nicotinic acid comprises the following steps:
[0033] (1) Preparation of methyl 5-(phthalamido)methylnicotinate
[0034] Add 0.1mol 5-hydroxymethyl nicotinic acid methyl ester, 0.6mol triphenylphosphine and 0.1mol phthalamide to a clean and dry three-necked flask, dissolve them in 20mL of tetrahydrofuran, add dropwise 0.6mol azo The ethyl acetate solution of diethyl diformate was refluxed for 3 hours, and the 5-hydroxymethyl nicotinic acid methyl ester was completely reacted to obtain 5-(phthalamide) methyl nicotinic acid methyl ester.
[0035] (2) Preparation of 5-(phthalamido)methylnicotinic acid
[0036] 5-(phthalamido) methyl nicotinic acid methanol solution, slowly dropwise add 1.0 M sodium hydroxide aqueous solution to adjust the pH of the reaction system to 12, stir for 24 hours, and the hydrolysis is complete by HPLC detection. Ethyl acetate was added for ex...
Embodiment 35
[0039] The preparation of embodiment 35-aminomethyl nicotinic acid
[0040] The preparation of 5-aminomethyl nicotinic acid comprises the following steps:
[0041] (1) Preparation of methyl 5-(phthalamido)methylnicotinate
[0042] Add 0.1mol 5-hydroxymethyl nicotinic acid methyl ester, 0.5mol triphenylphosphine and 0.12mol phthalamide to a clean and dry three-necked flask, dissolve them in 20mL of tetrahydrofuran, add dropwise 0.6mol azo The toluene solution of diethyl diformate was stirred at room temperature for 24 hours, and the 5-hydroxymethyl nicotinic acid methyl ester was completely reacted to obtain 5-(phthalamide) methyl nicotinic acid methyl ester.
[0043] (2) Preparation of 5-(phthalamido)methylnicotinic acid
[0044] 5-(phthalamido) methyl nicotinic acid ethanol solution, slowly dropwise add 1.0M sodium hydroxide aqueous solution to adjust the pH of the reaction system to 14, stir for 12 hours, and the hydrolysis is complete by HPLC detection. Ethyl acetate was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com