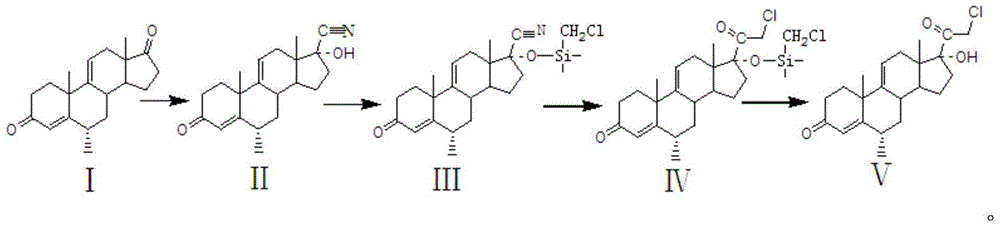
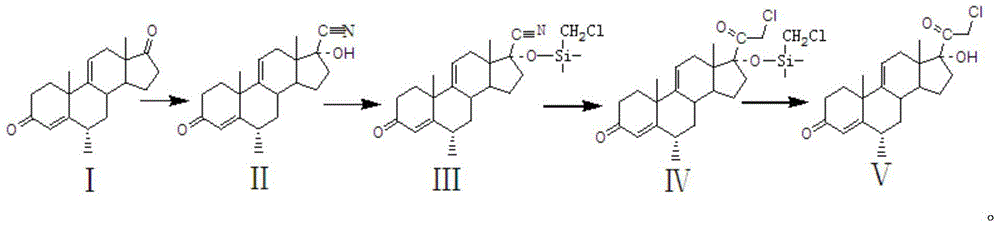
Preparation method of methylprednisolone key intermediate
A technology for methylprednisolone and intermediates, which is applied in the field of preparation of steroid drug intermediates, can solve the problems of complex reaction route, high production cost and high raw material cost, and achieves simple reaction, easy operation and high industrial value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] (1) Cyanohydrination reaction
[0013] Put 60 kg of compound Ⅰ, add 60 kg of acetone, 60 kg of tap water, 3 kg of triethylamine, 60 kg of potassium cyanide, heat up to 60°C for 15 hours, add 600 kg of water, stir for water analysis, centrifuge after water analysis, filter The cake was washed with water until neutral, and dried at 60°C to obtain 60 kg of compound II with a mass yield of 100%.
[0014] (2) Protection response
[0015] Take 60 kg of compound II, add 180 kg of dichloromethane and stir to dissolve completely, add 18 kg of imidazole, add 36 kg of trimethylchlorosilane dropwise at a temperature of 10°C, react at 25°C for 3 hours after the dropwise addition, add 18 kg of carbonic acid Potassium aqueous solution (potassium carbonate aqueous solution contains 0.6 kg of potassium carbonate and 17.4 kg of water), adjust the pH to neutral, add 600 kg of water to concentrate dichloromethane, water analysis, centrifugation, and dry the filter cake to obtain 75 kg of ...
Embodiment 2
[0021] (1) Cyanohydrination reaction
[0022] Put 60 kg of compound Ⅰ, add 240 kg of acetone, 0.6 kg of diisopropylamine, 6 kg of potassium cyanide, heat up to 50 ° C for 20 hours, add 600 kg of water, stir for water analysis, centrifuge after water analysis, wash the filter cake to medium After drying at 70°C, 59 kg of compound II was obtained with a mass yield of 98.3%.
[0023] (2) Protection response
[0024] Take 60 kg of compound II, add 120 kg of dichloromethane and stir to dissolve completely, add 6 kg of imidazole, add 36 kg of trimethylchlorosilane dropwise at 0°C, react at 0°C for 3 hours after the dropwise addition, add 18 kg of carbonic acid Sodium bicarbonate aqueous solution (sodium bicarbonate aqueous solution contains 0.6 kg of sodium bicarbonate and 17.4 kg of water), adjust the pH to 8. Dichloromethane was concentrated by adding 500 kg of water, analyzed in water, centrifuged, and the filter cake was dried to obtain 73 kg of compound III with a mass yield ...
Embodiment 3
[0030] (1) Cyanohydrination reaction
[0031] Put in 60 kg of compound I, add 6 kg of methanol, 60 kg of triethylamine, and 120 kg of potassium cyanide, heat up to 100 ° C for 1 hour, add 800 kg of water, stir for water analysis, centrifuge after water analysis, and wash the filter cake to medium properties, and dried at 70°C to obtain 59.4 kg of compound II with a mass yield of 99%.
[0032] (2) Protection response
[0033] Take 60 kg of compound II, add 600 kg of chloroform and stir to dissolve completely, add 60 kg of imidazole, add 36 kg of trimethylchlorosilane dropwise at a temperature of 50°C, react at 50°C for 1 hour after the dropwise addition, add 18 kg of pyridine , adjust the pH to 9. Add 500 kg of water to concentrate dichloromethane, water analysis, centrifugation, and dry the filter cake to obtain 75.3 kg of compound III, with a mass yield of 125.5%.
[0034] (3) Low temperature reaction
[0035] Take 75 kg of compound III, add 750 kg of tetrahydrofuran, coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com