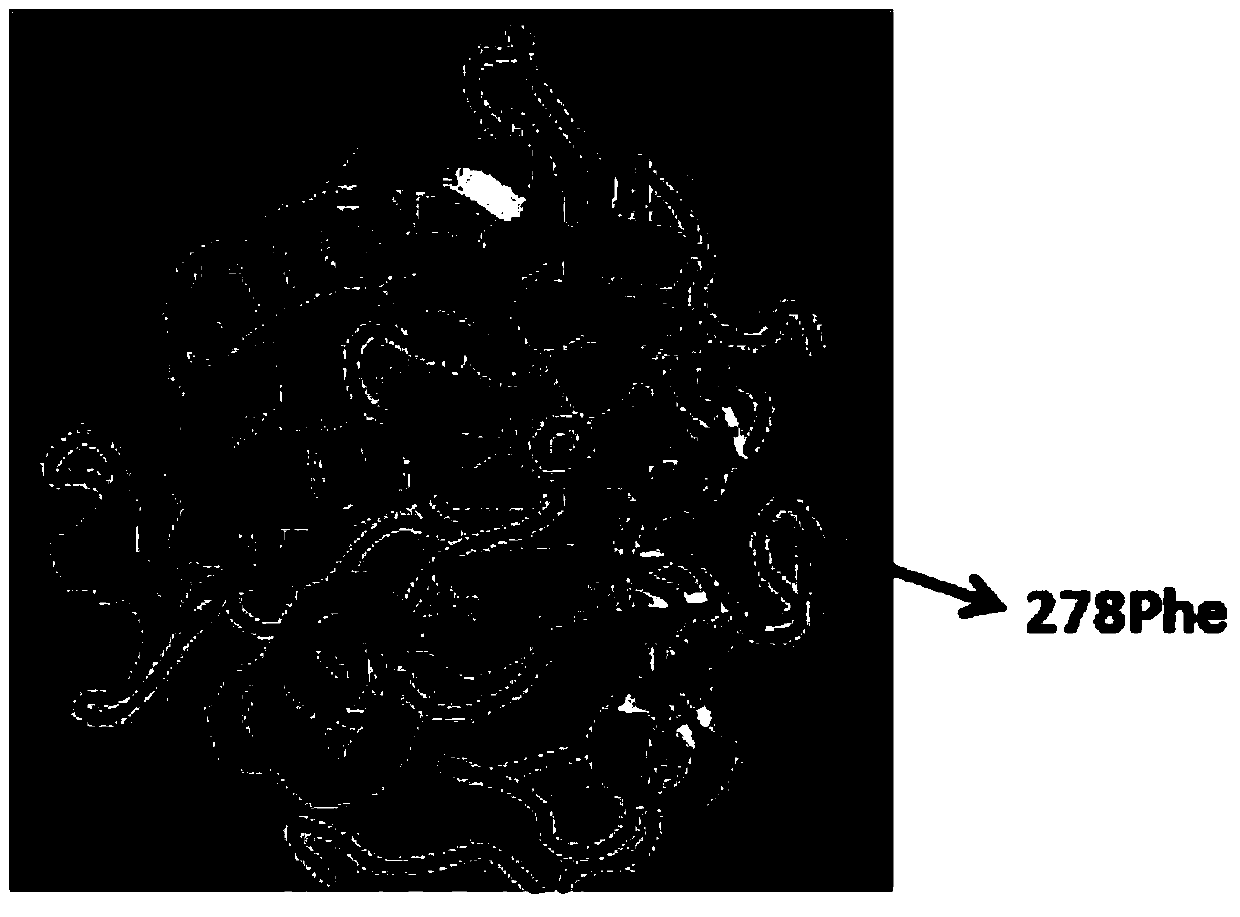
Higher-activity partial glyceride lipase mutant and application thereof
A partial glyceride and mutant technology, which is applied in the field of partial glyceride lipase mutants, can solve problems such as lack, increased cost input, and product price rise, and achieve good activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Construction of lipase SMG1 wild-type Pichia pastoris expression vector
[0036] According to Genbank's spherical Malassezia partial glycerol lipase smg1 gene (accession number: XM_001732152), Shanghai Sangon Bioengineering Co., Ltd. was entrusted to use the artificial synthesis method and optimize the sequence according to the codon preference of Pichia pastoris (sequence SEQ ID NO: 1). After gene synthesis, use primers SMG For: 5'-GGGGTACCAGCAGTATTTACGCCCGTGGCCG-3' and SMG Rev: 5'ACGCGTCGACTTAATGAGCACCAACCTGAGCT-3' to amplify the mature peptide sequence, PCR amplification conditions: 94°C for 5min; 94°C for 20s, 53°C for 30s, 72°C 80s at ℃, 25 cycles; 7min at 72℃. After the PCR amplification product was purified by the DNA purification kit, the purified gene fragment and plasmid pGAPZαA were digested with restriction enzymes Kpn I and Sal I respectively, ligated, and transformed into E. coli DH5α competent cells . Spread on LB (containing 25ug / ml zeocion) plate. T...
Embodiment 2
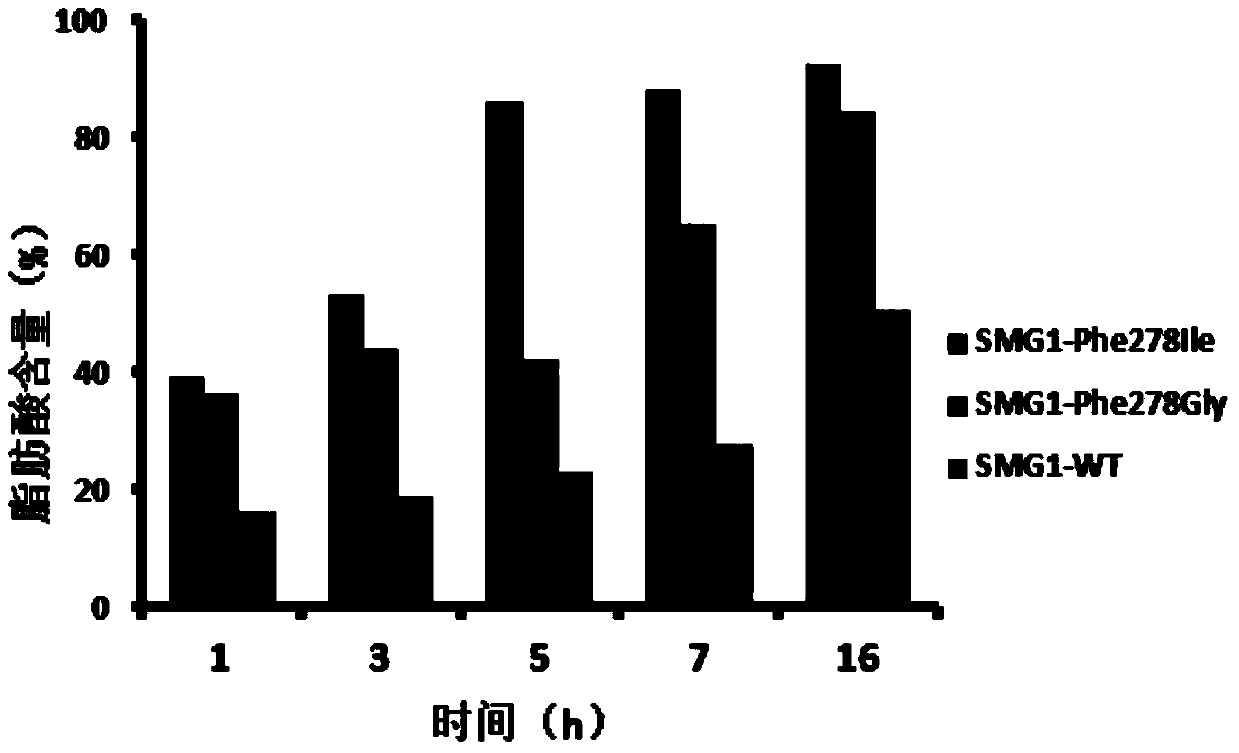
[0038] Construction of lipase SMG1278 amino acid site mutant
[0039] The mutants SMG1Phe278Gly and SMG1Phe278Ile were constructed by site-directed mutagenesis technology, and the target mutation sites were introduced by the overlap extension PCR method. Using the SMG1 wild-type gene pGAPZαA-SMG1 as a template, the site-directed mutagenesis reaction conditions were as follows:
[0040] Amplify the mutated upstream fragment:
[0041] 10X pfu buffer:
5
d NTP:
2.5
SMG for:
2.5
Phe278Ile rev:
2.5
Pfu DNA polymerase:
1.5
Double distilled water:
40
PGAPZαA-SMG1:
2
[0042] Amplify the mutated downstream fragment:
[0043] 10X pfu buffer
5
[0044] dNTPs:
2.5
Phe278Ile for:
2.5
3' AOX:
2.5
Pfu DNA polymerase:
1.5
Double distilled water:
40
PGAPZαA-SMG1:
2
[0045] Amplify full-length gene fragments with...
Embodiment 3
[0051] Recombinant expression and purification of lipase SMG1 and its mutants
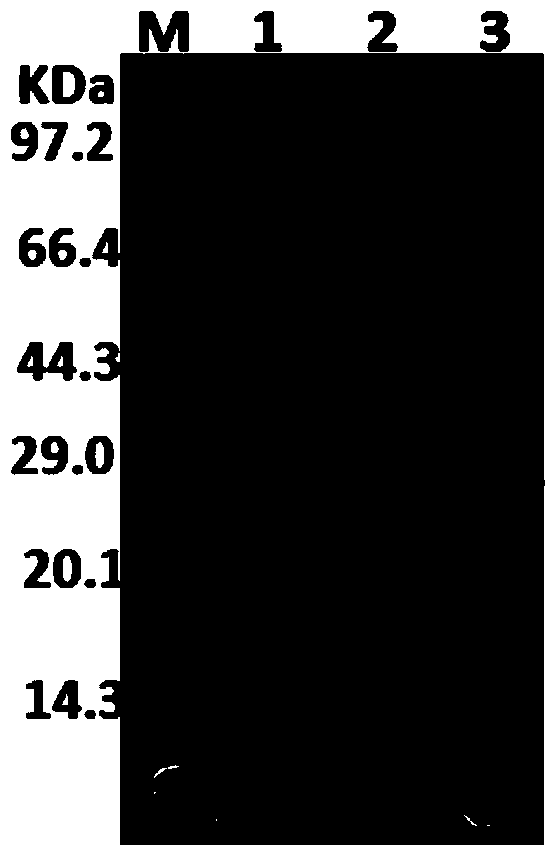
[0052] The mutant expression plasmid was linearized by the restriction endonuclease Bln I, and then electroporated into Pichia pastoris X-33 competent cells. Spread the transformation liquid on a YPD (100ug / ml Zeocin) plate, culture it at 30°C for 3 days, pick a single colony on the plate and ferment it in 100ml YPD medium for 72 hours, centrifuge and concentrate the fermentation liquid for SDS-PAGE detection, and obtain Recombinant strains capable of expressing lipase SMG1 mutant positive.
[0053] Inoculate the wild-type strain and each lipase mutant strain into 100ml of YPD medium, shake culture at 30°C and 200rpm for 48-72 hours, and collect the fermentation supernatant (10,000rpm, centrifuge for 20min, 4°C).
[0054] The fermentation supernatants of the SMG1 wild type and its mutant strains were suction-filtered with a 0.22um filter membrane. Afterwards, 10KD membrane cells (Vivaflow200, Sar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com