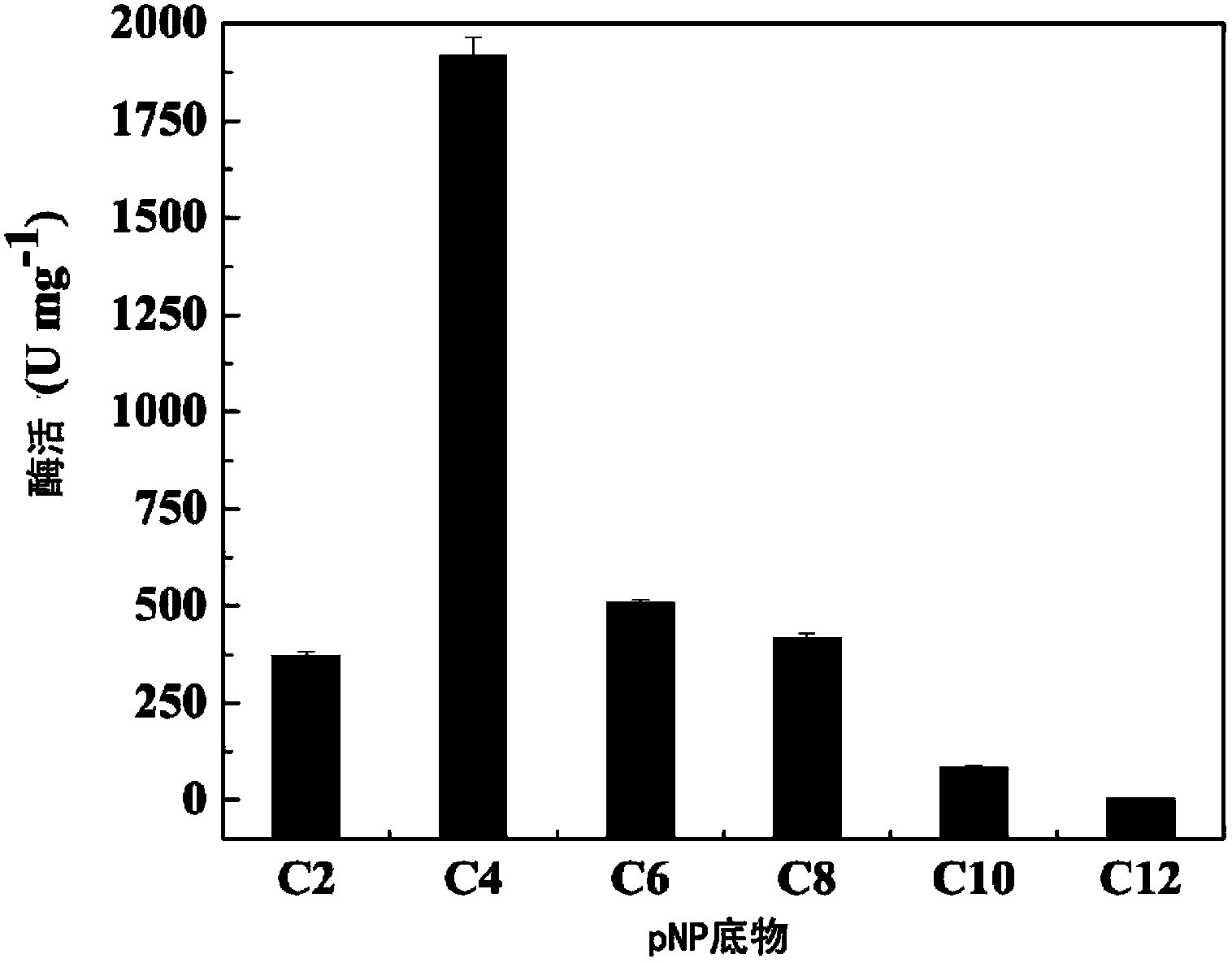
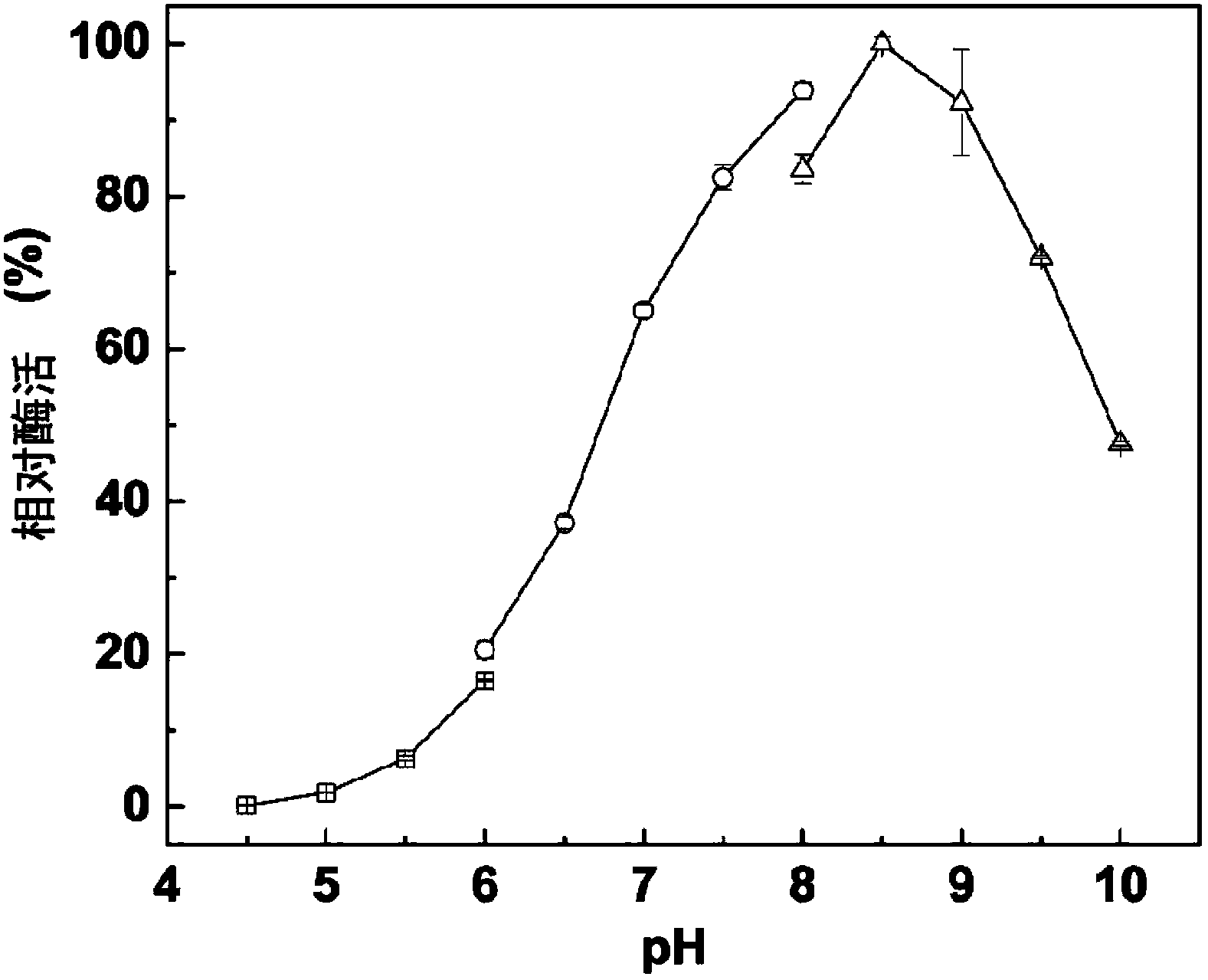
Tertiary alcohol ester hydrolase, encoding gene, carrier, engineering bacteria and application thereof
A technology of hydrolase and tertiary alcohol ester, applied in the direction of genetic engineering, hydrolase, application, etc., to achieve the effect of high resistance to organic solvents and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Acquisition of full-length esterase Est076 gene
[0068] Soil samples at 30 cm below the surface were collected in the Gaofeng Forest in West Hubei, Hangzhou, and the total metagenomic DNA was extracted using the Mo Bio Power Soil DNA isolation kit (M) (Carlsbad, CA) kit, and the Copy Control Fosmid Library production kit (Epicentre, USA) library construction kit to construct environmental metagenomic library. The metagenomic DNA of the library was screened by esterase using the plate substrate transparent circle method, and the surrounding hydrolysis was obtained by screening with Luria-Bertani (LB) solid medium containing 1% tributyrin substrate (polyvinyl alcohol emulsion). Positive clones with transparent circles. High-copy induction of the Fosmid recombinant plasmid in the positive clones (see the Copy Control Fosmid Library production kit manual for specific methods), and then extract the Fosmid recombinant plasmid. Use endonuclease Sau3A I to inco...
Embodiment 2
[0069] Embodiment 2: Construction of the expression vector system containing esterase Est076 target gene
[0070] According to the full-length gene sequence of esterase Est076, the upstream primer Est076F (5′-CCG) was designed to amplify the complete coding reading frame GAATTC ATGAGTCAGCTTCCAGCAG-3′) and the downstream primer Est076R (5′-GCG GCCGCC TTCAAATACTTGTCC-3'), respectively introduce restriction endonuclease sites on the upstream and downstream primers (upstream introduced enzyme cutting site is EcoR I, downstream is Not I). Taking the plasmid containing the esterase gene sequence described in the present invention as a template, after PCR amplification, use the esterase Est076 nucleotide fragment obtained by restriction endonuclease EcoR I and Not I double enzyme digestion amplification, ligation To the expression vector pET21a with corresponding cuts (digested by EcoR I and Not I), transformed into E.coil DH5α, the recombinant expression vector was identified und...
Embodiment 3
[0071] Embodiment 3: Expression and purification preparation of esterase Est076
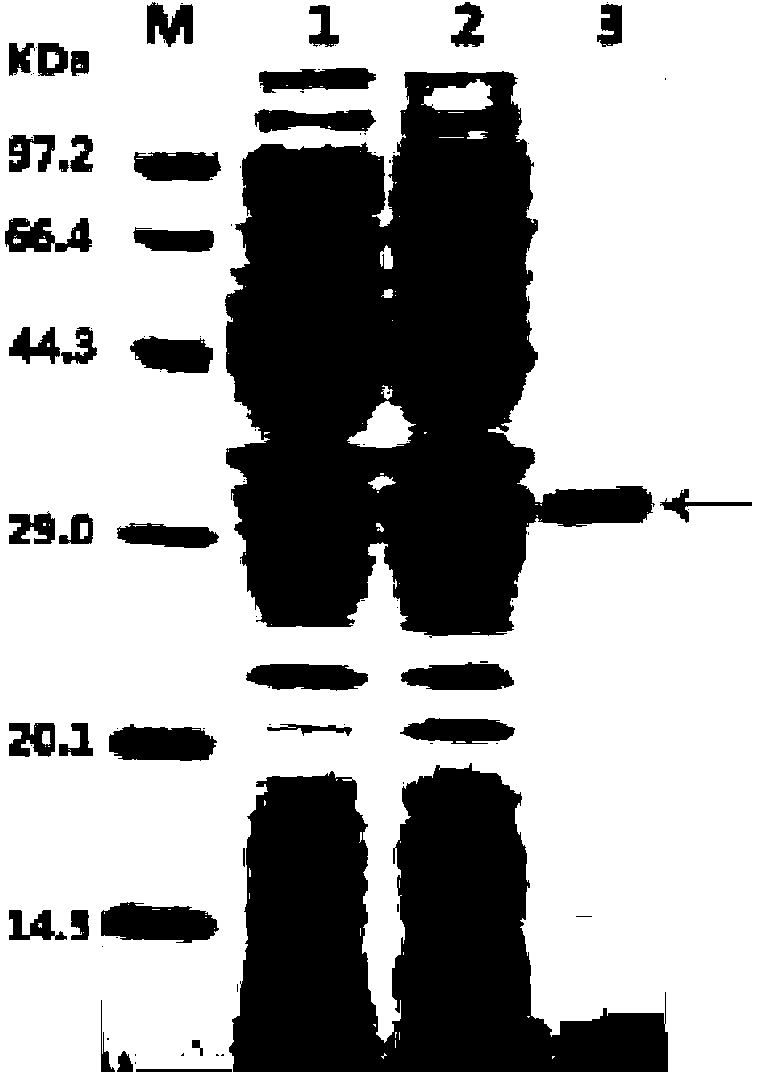
[0072] The esterase expression system E.coilBL21(DE3) / pET21a / Est076 obtained in Example 2 was inoculated into Luria-Bertani (LB) liquid medium containing 100 μg / ml ampicillin (Sigma, USA), and cultured at 37°C to logarithmic growth phase (OD 600After adding IPTG (genview, USA) with a final concentration of 0.15mM, induce culture at 15°C for about 20 hours, collect the bacteria by centrifugation, resuspend in 50mM Tris-HCl (pH8.0) buffer, and ultrasonically break The bacterial suspension was centrifuged to remove the precipitate, and the supernatant was obtained as a crude enzyme solution containing esterase protein. The crude enzyme solution was filtered through a 0.45 μm cellulose acetate filter, followed by nickel ion affinity chromatography to obtain the pure enzyme protein (SEQ ID No.1) of esterase Est076 described in the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com