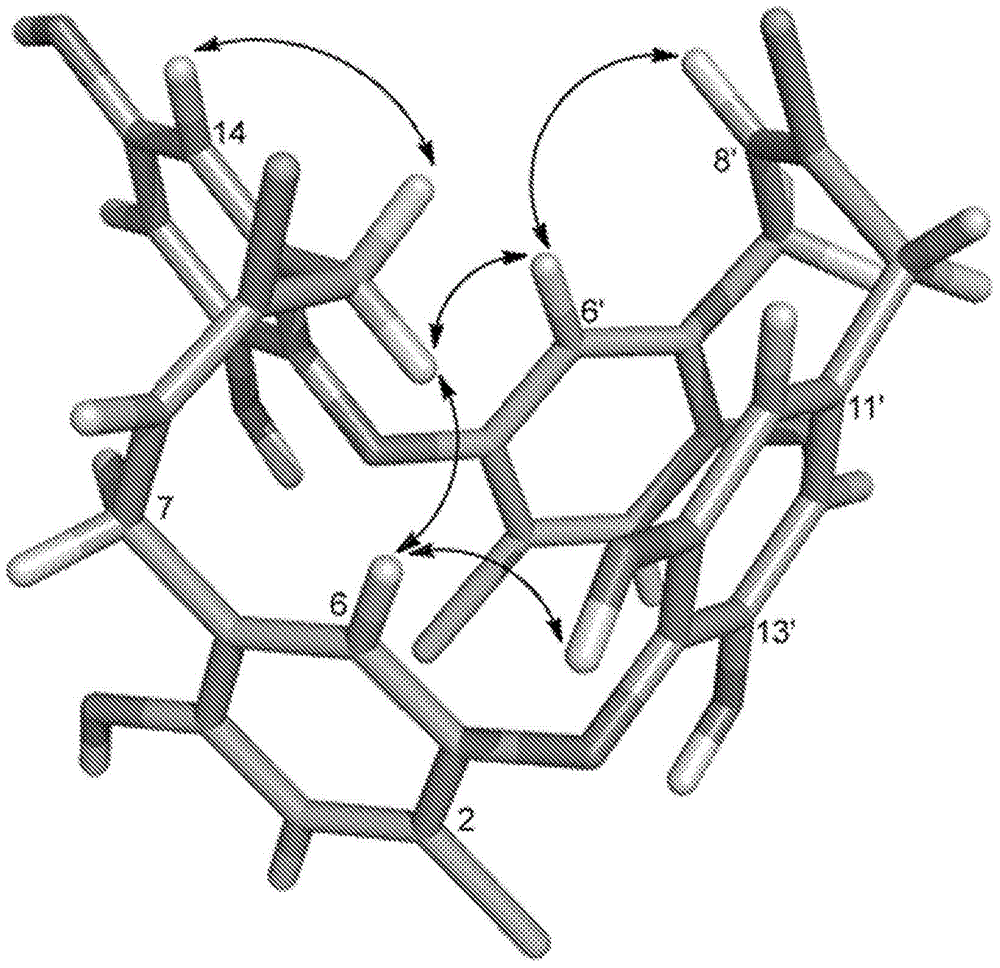Christine analogues that inhibit the FTSZ protein
A technology of drugs and compounds, applied in the field of antimicrobial compounds, which can solve problems such as the rising prevalence of invasive infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Compound Isolation and Structure Determination
[0217] Samples of the Chrysophaeum taylori algae were collected from 20 ft below the surface in Round Bay, St. John's Island (Lewis and Bryan, Am. J. Bot., 28:343, 1941). The algae, which is found growing in fluffy colonies on coarse sand or crushed coral substrates, exhibits a sulfur yellow color and can turn rusty brown within seconds when disturbed. The multicellular structure of C. taylori is extremely fragile, and the sticky cells are not well preserved. However, when freshly collected or freshly preserved (2.5% glutaraldehyde in seawater) samples were viewed under a light microscope, stalk-like structures consisting of branched slime "ribbons" of pyriform embedded cells could be seen. In addition, algae collections contained the known styrylchromone hormothamnione and showed remarkable similarities to field records published by Gerwick (J. Nat. Prod., 52:252, 1989) at all stages of manipulation.
[0218] Antimicro...
Embodiment 2
[0253]Antimicrobial tests of compounds 1, 1a, 4-8
[0254] Compounds 1-8 were tested against Staphylococcus aureus (SA, ATCC25923), methicillin-resistant Staphylococcus aureus (MRSA, ATCC BAA-41), Antimicrobial activity of multidrug-resistant Staphylococcus aureus (MDRSA, ATCC BAA-44), Enterococcus faecium (EF, ATCC49032), vancomycin-resistant Enterococcus faecium (VRE, ATCC700221) and Bacillus subtilis (ATCC49343). For the plate diffusion assay, agar plates inoculated with the bacterial suspension were prepared by adding 500 μL of the bacterial culture grown for 24 h to 100 mL of autoclaved and cooled to 55° C. Mueller Hinton II agar. The inoculated liquid agar (10 mL) was immediately transferred to a square petri dish and allowed to cool for 1 h. Control drugs for each microorganism included kanamycin (50 μg) for S. aureus and nitrofurantoin (25 μg) for MRSA. After incubation for 18 h at 37°C, the zone of inhibition was measured. The MICs of compounds 1 and 4-8 were deter...
Embodiment 3
[0262] GTPase activity and FtsZ polymerization
[0263] The bacterial cytoskeletal protein FtsZ is a GTPase and shares structural homology with the eukaryotic cytoskeletal protein tubulin, but lacks significant sequence similarity. FtsZ is essential for bacterial cell division. Non-cleavable GTP analogs have been reported to inhibit FtsZ in vitro, as do several small molecules. Inhibition of FtsZ terminates bacterial cell division and is a potent target for new antimicrobial agents. Compound 1 was tested for its ability to inhibit the GTPase activity of recombinant Escherichia coli FtsZ using a colorimetric assay that detects inorganic molybdenum released upon hydrolysis of GTP to GDP by complexation with molybdate (also known as malachite green). Phosphate (P i ). GTPase assay in 50mM MES, pH6.5, 50mM KCI, 5mMMgCl 2 in the presence of 2 μM FtsZ and different concentrations of compound 1. The solution was incubated at room temperature for 5 min before adding 0.25 mM GTP....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com