Preparation method of polymer-bioglass hybrid material for bone repair
A bioglass and hybrid material technology, applied in medical science, prosthesis, etc., can solve the problems of lack of grafting bioactive molecules, difficulty in regulating cell behavior, etc., and achieve the effect of promoting proliferation and differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
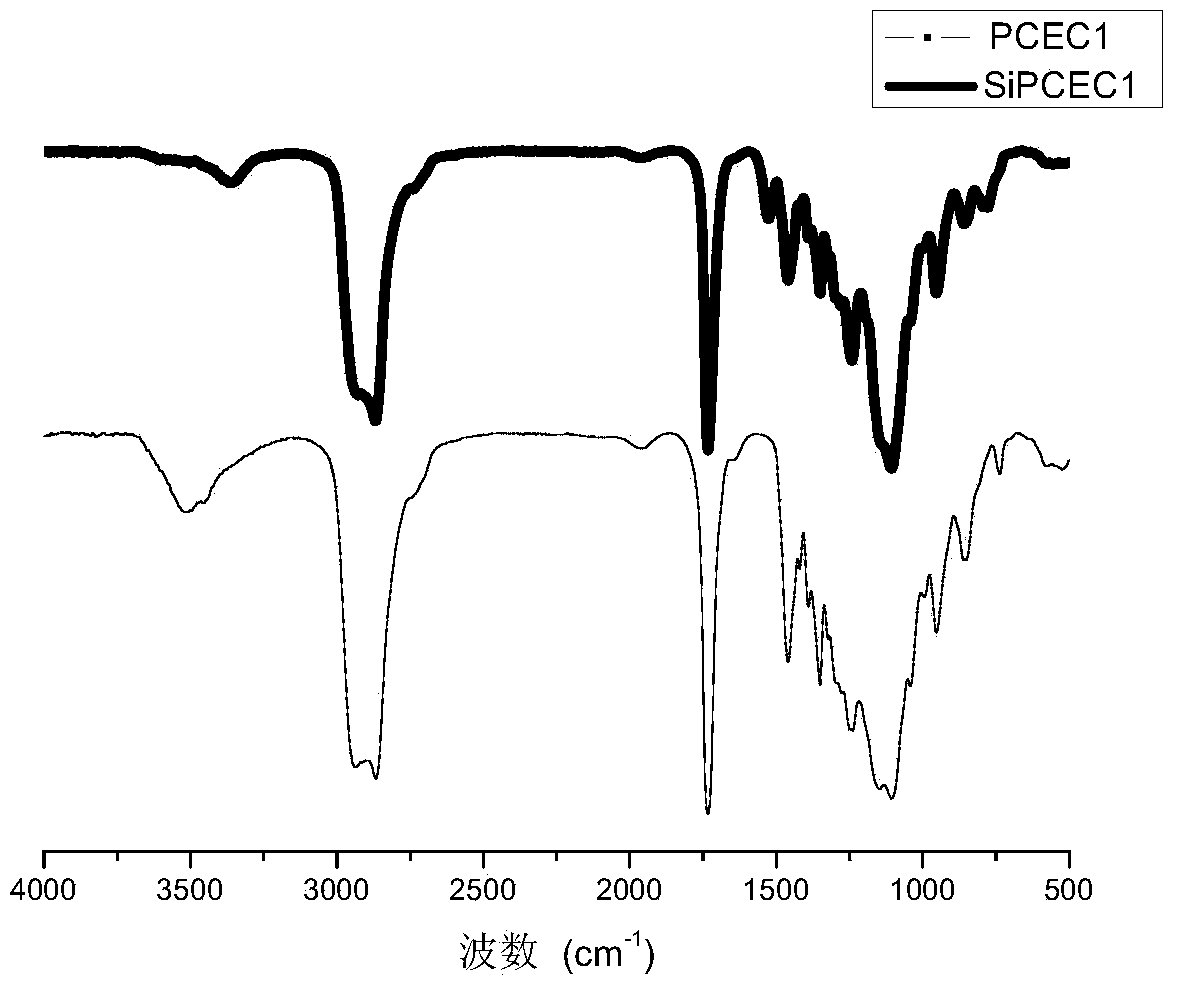
[0034] Add a mixture of α-propargyl-ε-caprolactone and caprolactone to 3 g of polyethylene glycol 2000 with both terminal groups being hydroxyl groups (α-propargyl-ε-caprolactone and caprolactone The molar ratio is 1:5) 3mL, then add 50μL of 1mol / L stannous octoate and react at 140°C for 16h. After rotary evaporation, polycaprolactone-polyethylene glycol- Polycaprolactone triblock copolymer. Dissolve the above three-block copolymer in DMF, add 0.01 g of cuprous bromide after adding the azidized RGD at the end group, and obtain a three-block copolymer with RGD as the side chain. Add 1.482 g of isocyanate propyltriethoxysilane to the tri-block copolymer whose side chain is RGD, and react at 70° C. for 24 hours to obtain a tri-block copolymer whose terminal group is triethoxysilyl. Add 4.765g TEOS, 1.647g PH=3 hydrochloric acid aqueous solution, 2.1045g ethanol, 0.2778g TEP, 3.2413g calcium nitrate to the tri-block copolymer whose terminal group is triethoxysilyl group. After t...
Embodiment 2
[0039]Add a mixture of α-propargyl-ε-glycolide and glycolide to 6 g of polyethylene glycol 4000 with hydroxyl groups at both ends (α-propargyl-ε-glycolide and glycolide The molar ratio is 1:10) 12mL, then add 1mol / L stannous octoate 100μL and react at 120°C for 6h. After the reaction is completed, add 10-20mL of petroleum ether to wash, and obtain the propargyl functional group of polycaprolactone-polyethylene glycol-polycaprolactone triblock copolymer. The tri-block copolymer was dissolved in DMF, and 0.01 g of cuprous bromide was added after adding the azide-terminated RGD to obtain a tri-block copolymer whose side chain was RGD. Add 0.741 g of isocyanate propyltriethoxysilane to the tri-block copolymer, and react at 70° C. for 24 hours to obtain a tri-block copolymer whose terminal group is a triethoxysilyl group. Add 4.765g TEOS, 1.647g PH=3 hydrochloric acid aqueous solution, 0.2778g TEP, 3.2413g calcium nitrate to this polymer. Then add 0.2mL of 0.1mol / L hydrochloric a...
Embodiment 3
[0041] Add the mixed solution of α-propargyl lactide and lactide (the mol ratio of α-propargyl lactide and lactide is 10: 1) 1.5mL of the mixed solution, then add 30μL of 1mol / L stannous octoate, and react at 160°C for 16h. After the reaction is completed, add 10-20mL of petroleum ether to wash, and obtain the propargyl functional group after rotary evaporation Polylactic acid-polyethylene glycol-polylactic acid triblock copolymer. The functional polymer was dissolved in DMF, and 0.01 g of cuprous bromide was added after adding the azidized RGD at the end group to obtain a tri-block copolymer whose side chain was RGD. Add 0.741 g of isocyanate propyltriethoxysilane to the tri-block copolymer, and react at 60° C. for 24 hours to obtain a tri-block copolymer whose terminal group is a triethoxysilyl group. Add 4.765g TEOS, 1.647g PH=5 hydrochloric acid aqueous solution, 2.1045g ethanol, 0.2778g TEP, 3.2413g calcium nitrate to this polymer. Then add 0.2mL of 0.1mol / L hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive modulus | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com