Method for using hydrocyanic acid mixed gas to prepare 2-hydroxy-4-methylthio-butyronitrile and continuous production method for 2-hydroxy-4-methylthio-butyronitrile
A technology of methylthiobutyronitrile and hydrocyanic acid, applied in the field of chemical industry, can solve the problems of high energy consumption of 3-methylthiopropanal, loss of 3-methylthiopropanal, cost increase, etc., and achieve hydrogen cyanide The effect of high acid utilization rate, lower production cost and higher production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
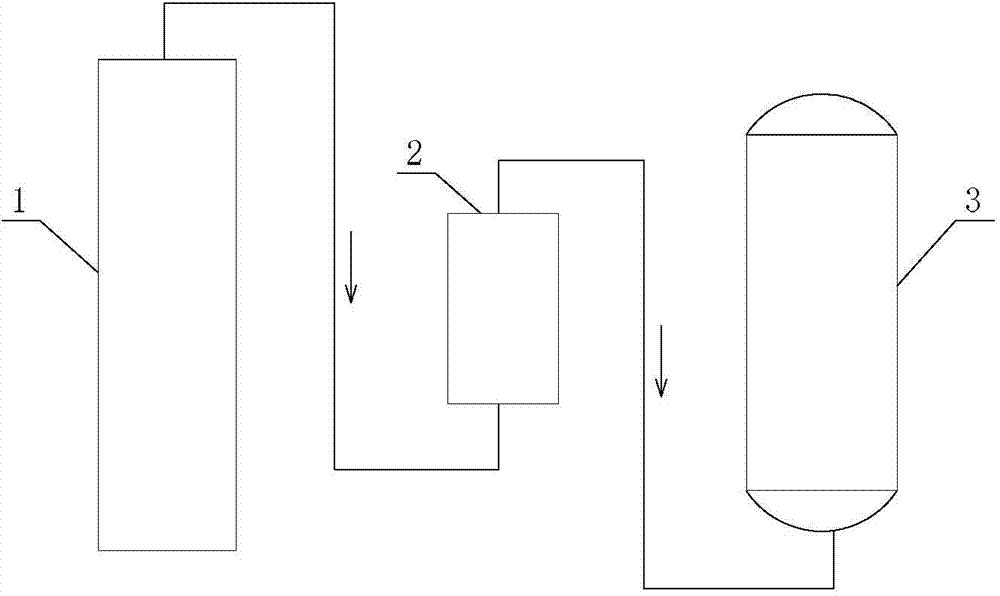
[0052] The hydrocyanic acid mixed gas I from the hydrocyanic acid synthesis tower was detected, and the composition of the hydrocyanic acid mixed gas I was: 8.87% hydrocyanic acid gas, 3.88% water vapor, 1.64% ammonia gas, 1.13% hydrogen gas, nitrogen gas 76.01%, oxygen 1.48%, carbon monoxide 5.67%, carbon dioxide 1.13%, methane 0.39%.
[0053] Hydrocyanic acid mixed gas I passes through a 75% sulfuric acid tower to absorb ammonia and water vapor in the mixed gas, and the composition of the obtained hydrocyanic acid mixed gas II is: 9.35% hydrogen cyanide gas, 1.57% hydrogen gas, and 79.44% nitrogen gas %, oxygen 1.71%, carbon monoxide 5.79%, carbon dioxide 1.50%, methane 0.64%.
Embodiment 2
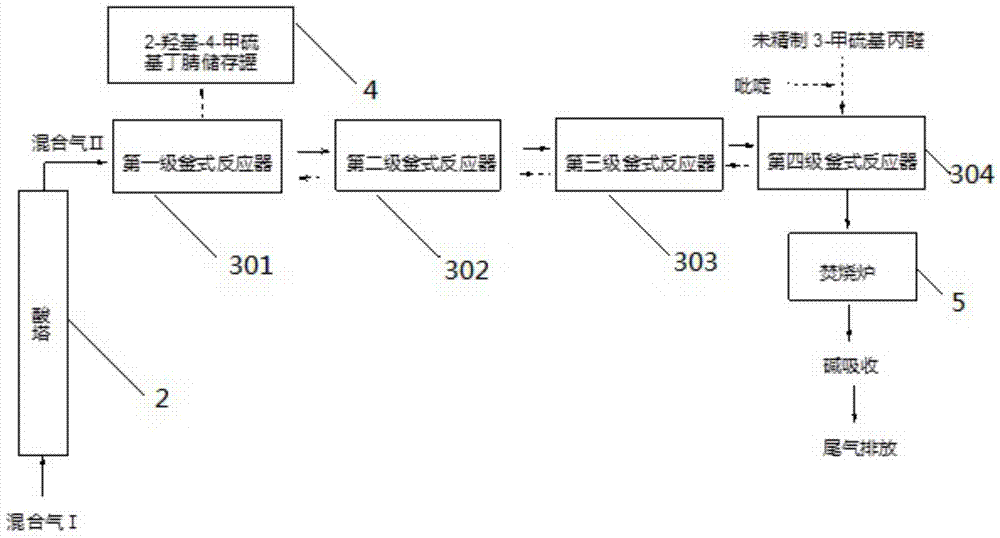
[0055] Pass hydrogen cyanide mixed gas II into 223.3g of 94.5% methional, which contains 3.3g of pyridine. React under normal pressure, control the reaction temperature to 45°C, the ventilation rate to 300L / min, absorb the tail gas with sodium hydroxide, and monitor the residual amount of methionaldehyde by HPLC. When the residual amount of methionaldehyde is less than 0.5%, it is the end of the reaction, and the feeding can be stopped. A total of 270.64 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.5%.
Embodiment 3
[0057] Pass hydrogen cyanide mixed gas II into 223.3g of 94.5% methional, which contains 2.2g of pyridine and 10g of water. Under 0.15MPa, the reaction temperature is controlled to be 42°C, the ventilation rate is 280L / min, the tail gas is absorbed with sodium hydroxide, and the residual amount of methional is monitored by HPLC. When the residual amount of methionaldehyde is less than 0.5%, it is the end of the reaction, and the feeding can be stopped. A total of 279.54 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.07%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com