Ion-exchange membrane, manufacturing method of ion-exchange membrane, and electrolyzer
An ion exchange membrane and ion exchange technology, applied in chemical instruments and methods, organic diaphragms, synthetic resin layered products, etc., can solve the problem of no cathode activity, and achieve the effect of stable electrolytic performance and less impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
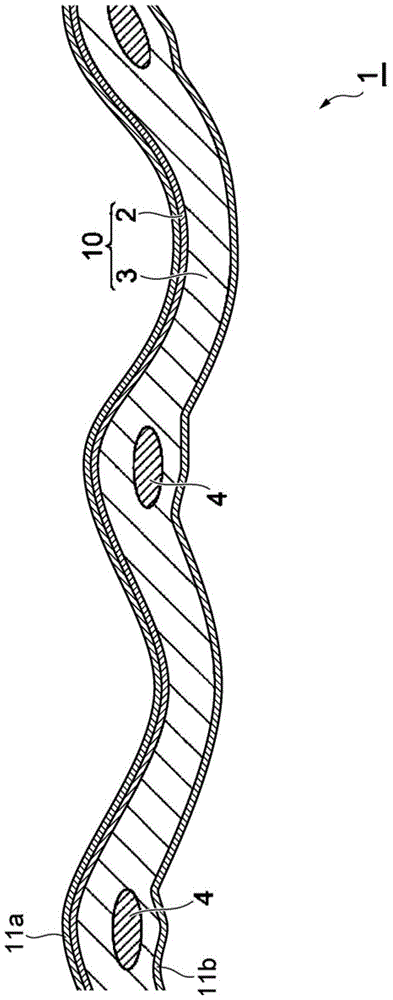
[0197] As a reinforcing core material, a polytetrafluoroethylene (PTFE) 100-denier flat yarn twisted at 900 times / m was used (hereinafter referred to as PTFE thread). As the sacrificial yarn of the warp, a polyethylene terephthalate (PET) with 35 denier and 8 filaments twisted 200 times / m (hereinafter referred to as PET yarn) was used. In addition, as the sacrificial yarn of the weft, a polyethylene terephthalate (PET) with 35 denier and 8 filaments twisted at 200 twists / m was used. First, the number of PTFE threads was 24 / inch, and two sacrificial threads were arranged between adjacent PTFE threads to perform flat weaving to obtain a woven fabric with a thickness of 100 μm.
[0198] Next, using CF 2 =CF 2 with CF 2 =CFOCF 2 CF(CF 3 )OCF 2 CF 2 COOCH 3 The copolymer prepared ion exchange capacity is the polymer (A1) of dry resin of 0.84mg equivalent / g, utilizes CF 2 =CF 2 with CF 2 =CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 Copolymer of F A polymer (B1) of dry resin hav...
Embodiment 2
[0206] In Example 1, the dispersion by the ball mill was adjusted until the average particle diameter of the zirconia particles in the suspension was 1.12 μm to obtain a suspension; except that, an ion exchange membrane was produced in the same manner as in Example 1. .
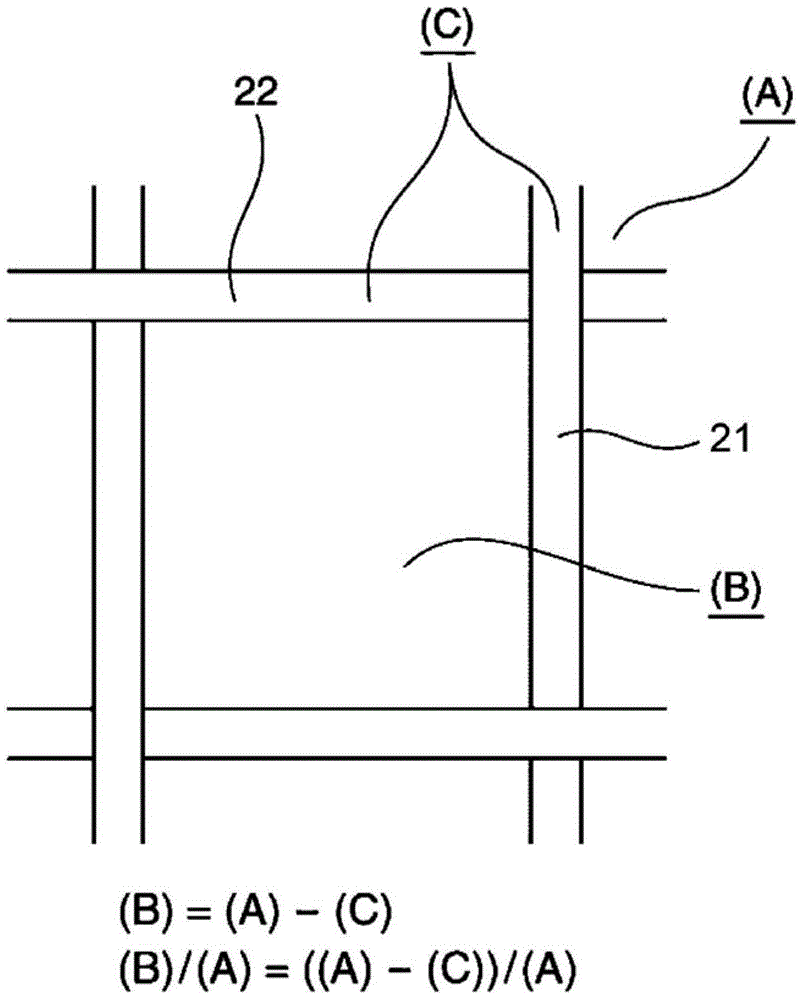
[0207] The dried coating of the ion-exchange membrane was measured by fluorescent X-ray measurement, and the coating density was measured per 1 cm 2 0.5mg in. In addition, the specific surface area of the coating obtained by scattering measured by SAXS was 2.2m 2 / g.
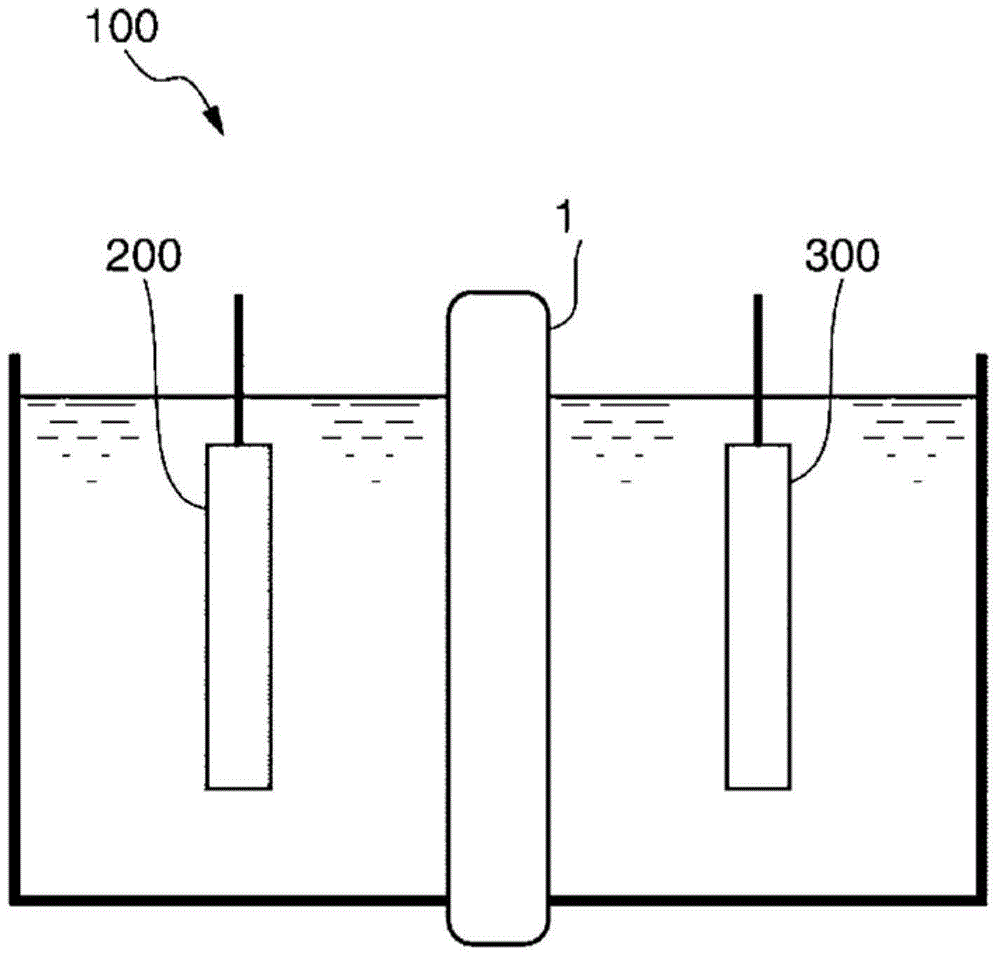
[0208] The ion-exchange membrane with the dry coating was wetted with 2% by weight of sodium bicarbonate, and then the ion-exchange membrane was used to measure the tolerance of impurities in finite-gap electrolysis. As a result, the reduction in current efficiency was 0.27% / day, showing relatively High impurity durability. The results are listed in Table 1.
Embodiment 3
[0210] In Example 1, the zirconia particles were changed to particles with a primary particle diameter of 2.50 μm, and the dispersion by a ball mill was adjusted until the average particle diameter of the zirconia particles in the suspension was 1.80 μm to obtain a suspension. ; Except that, an ion exchange membrane was produced in the same manner as in Example 1.
[0211] The dried coating of the ion-exchange membrane was measured by fluorescent X-ray measurement, and the coating density was measured per 1 cm 2 0.5mg in. In addition, the specific surface area of the coating obtained by scattering measured by SAXS was 1.8m 2 / g.
[0212] The ion-exchange membrane with the dry coating was wetted with 2% by weight of sodium bicarbonate, and then the ion-exchange membrane was used to measure the tolerance of impurities in finite-gap electrolysis. As a result, the reduction in current efficiency was 0.24% / day, showing Higher impurity durability. The results are listed in Tab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com