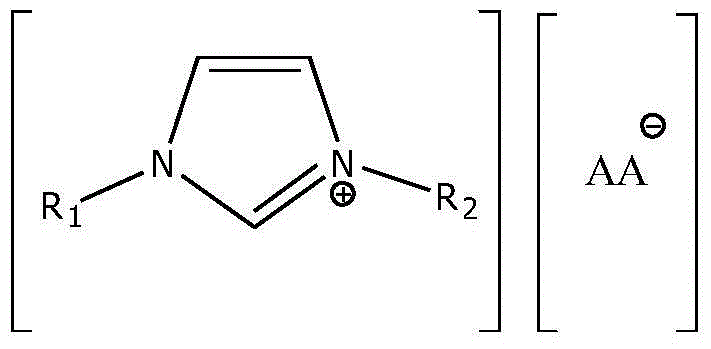
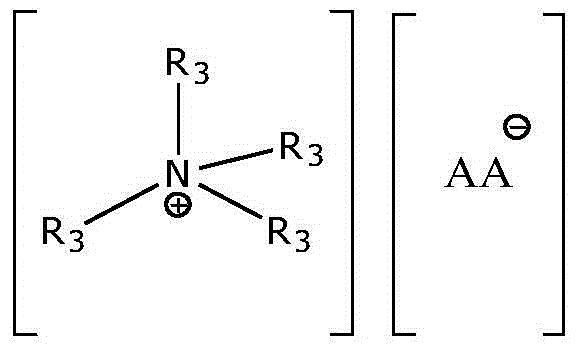
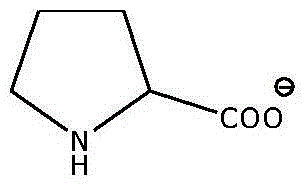
A kind of preparation method of proline ionic liquid catalytic synthesis dibutyl carbonate
A technology of dibutyl carbonate and ionic liquids, which is applied in the field of preparation of proline ionic liquids to catalyze the synthesis of dibutyl carbonate, can solve problems such as complex operating procedures, general catalytic effect, and difficulty in large-scale industrial production, and achieve operational The effect of simple process, reduced energy consumption and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 50 mL reactor, after replacing the air in the reaction vessel with an inert gas, add 5.92 g (80 mmol) of n-butanol, 1.8 g (20 mmol) of dimethyl carbonate and ionic liquid N,N,N,N '-Tetraethylquaternary ammonium L-proline salt 0.15 g (2 wt% of the total mass of raw materials), after stirring and mixing uniformly, heated to a reaction temperature of 110 ° C, carried out transesterification for 4 h, after the reaction was completed, the reaction The feed liquid is cooled in a phase separator, static phase separation, and the separated product and ionic liquid catalyst are poured. The used ionic liquid can be used for the next reaction after vacuum distillation. The product is analyzed by gas chromatography, and dimethyl carbonate The conversion rate of ester is 99.0%, and the selectivity of dibutyl carbonate is 95%.
Embodiment 2
[0028] In a 50 mL reactor, after replacing the air in the reaction vessel with an inert gas, add 5.92 g (80 mmol) of n-butanol, 1.8 g (20 mmol) of dimethyl carbonate and ionic liquid N,N,N,N '-Tetraethylquaternary ammonium L-homoproline salt 0.12 g (1.5 wt% of the total mass of raw materials), after stirring and mixing uniformly, heated to a reaction temperature of 110 ° C, and carried out transesterification for 4 h. The reaction feed liquid is cooled in a phase separator, static phase separation, and the separated product and ionic liquid catalyst are poured. The used ionic liquid can be used for the next reaction after vacuum distillation. The product is analyzed by gas chromatography. The conversion rate of methyl ester is 98.9%, and the selectivity of dibutyl carbonate is 94.8%.
Embodiment 3
[0030] In a 100 mL reactor, after replacing the air in the reaction vessel with an inert gas, add 7.4 g (100 mmol) of n-butanol, 1.8 g (20 mmol) of dimethyl carbonate and ionic liquid N,N,N,N '-Tetraethylquaternary ammonium L-hydroxyproline salt 0.28 g (3 wt% of the total mass of raw materials), after stirring and mixing evenly, heated to a reaction temperature of 110 ° C, and carried out transesterification for 5 h. The reaction feed liquid is cooled in a phase separator, static phase separation, and the separated product and ionic liquid catalyst are poured. The used ionic liquid can be used for the next reaction after vacuum distillation. The product is analyzed by gas chromatography. The conversion rate of methyl ester is 96.9%, and the selectivity of dibutyl carbonate is 92.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com