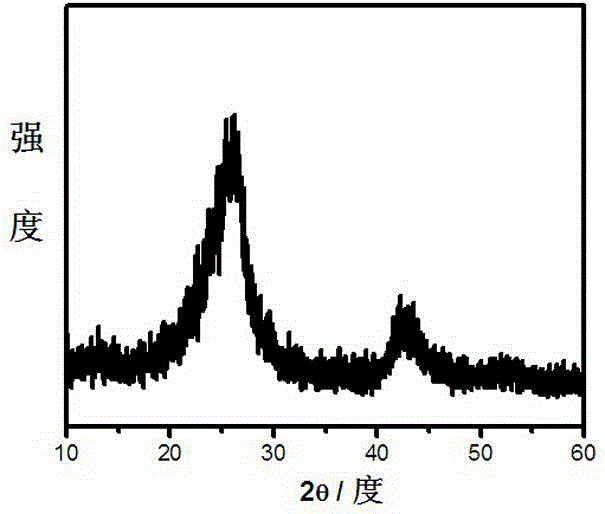
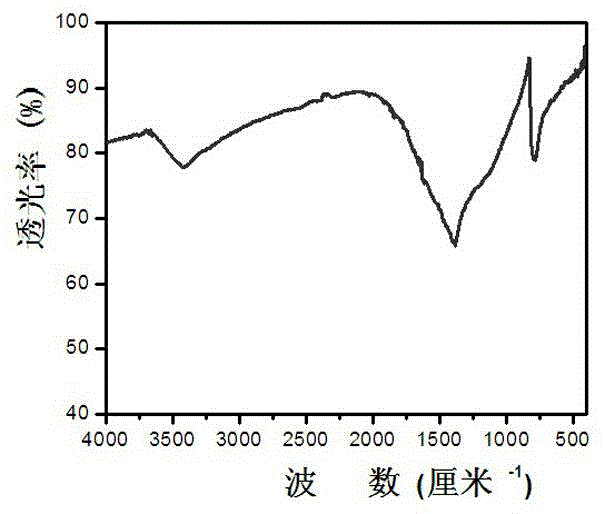
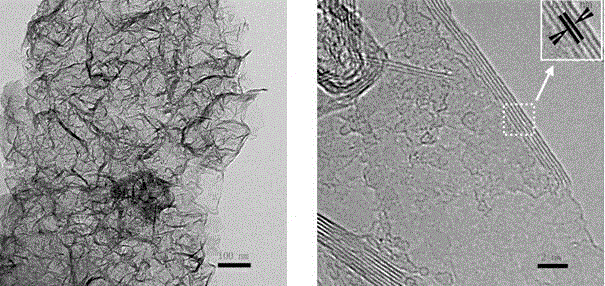
A non-metallic material for efficient visible light-driven catalytic water splitting
A non-metallic material, catalytic decomposition technology, applied in the field of material preparation and application, can solve the problems of high cost, inefficiency, environmental pollution, etc., and achieve the effects of resistance to mechanical wear, low cost, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation steps of the present invention are as follows:
[0019] After boron oxide, urea, and glucose are mixed and ground evenly in a mass ratio of 1:2:0.3, the mixed sample is taken in a corundum magnetic boat, and then placed in a horizontal high-temperature tube furnace. Raise the temperature at 5-10 ℃ / min to 1250 ℃ and keep it for 4-8 hours; take out the sample and wash it with 0.1mol / L dilute hydrochloric acid, centrifuge and dry to obtain graphite phase boron carbon nitrogen powder. Accurately weigh 50 mg of the prepared powder catalyst and place it in a photo-splitting water reactor to test the performance of photo-splitting water to produce hydrogen and oxygen.
Embodiment 1
[0021] After boron oxide, urea, and glucose are mixed and ground evenly in a mass ratio of 1:2:0.3, the mixed sample is taken in a corundum magnetic boat, and then placed in a horizontal high-temperature tube furnace. The temperature was raised to 1250°C at a speed of 5°C / min and kept for 4 hours; the sample was taken out and washed with 0.1mol / L dilute hydrochloric acid, centrifuged and dried to obtain graphite phase boron carbon nitrogen powder. Accurately weigh 50 mg of the prepared powder catalyst and place it in a photo-splitting water reactor to test the performance of photo-splitting water to produce hydrogen and oxygen.
Embodiment 2
[0023] After boron oxide, urea, and glucose are mixed and ground evenly in a mass ratio of 1:2:0.3, the mixed sample is taken in a corundum magnetic boat, and then placed in a horizontal high-temperature tube furnace. The temperature was raised to 1250°C at a speed of 10°C / min and kept for 8 hours; the sample was taken out and washed with 0.1mol / L dilute hydrochloric acid, centrifuged and dried to obtain graphite phase boron carbon nitrogen powder. Accurately weigh 50 mg of the prepared powder catalyst and place it in a photo-splitting water reactor to test the performance of photo-splitting water to produce hydrogen and oxygen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com