Hyperbranched polyurethane-acrylate UV (Ultraviolet) light cured resin
A polyurethane acrylate and light-curing resin technology, which is applied in the direction of polyurea/polyurethane coatings, polyurea/polyurethane adhesives, adhesive types, etc., can solve the problems of non-acrylic double bonds and inability to be light-cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
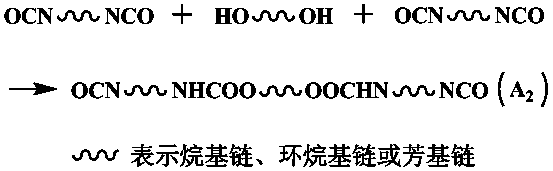
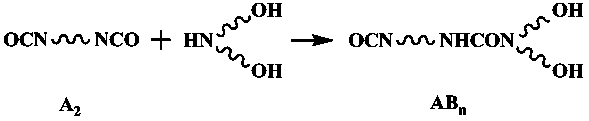
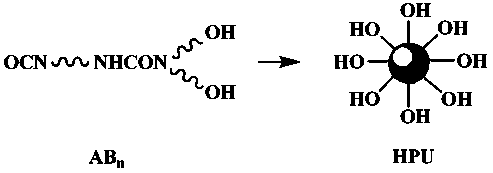
[0017] Add 6.75g of 1,4-butanediol (BDO) and 20g of N, N-dimethylformamide (DMF) to 33.344g of isophorone diisocyanate (IPDI) under stirring at 50°C. Reaction 5 Hours, a colorless transparent liquid was obtained; the reaction system was cooled to 0°C, and a mixture of 7.89g diethanolamine (DEOA) and 20g N, N-dimethylformamide (DMF) was added to the reaction system and reacted for 3 hours , to obtain a colorless transparent liquid; control the reaction conditions, add capping after 3 hours of reaction, separate and dry to obtain white powder hyperbranched polyurethane.
[0018] 44.458g IPDI and 50g DMF were added under stirring at 50°C, 23.224g HEA and 30g DMF solution were added dropwise to the constant pressure dropping funnel, and the reaction was carried out for 4 hours to obtain a semi-adduct of IPDI / HEA.
[0019] According to the remaining -OH content in the hyperbranched polyurethane, add IPDI / HEA in an equimolar amount, raise the temperature to 40°C, react for 4 hours a...
Embodiment 2
[0022] Dissolve 26.123g of toluene diisocyanate (TDI) in 30g of N,N-dimethylformamide (DMF), add 30g of polyethylene glycol 400 (PEG 400) and 10g of N,N-dimethylformamide (DMF) under stirring at 25°C A mixture of formamide (DMF) was reacted at constant temperature for 3 hours to obtain a yellowish transparent liquid; the system was cooled to -10°C, and 7.89g of diethanolamine (DEOA) and 20g of N,N-dimethylformamide (DMF ) was added to the reaction system and reacted for 3 hours to obtain a yellowish transparent liquid; raise the temperature to 40°C and react for 2.5 hours to end-cap, separate and dry to obtain a white powder hyperbranched polyurethane acrylate UV light Cured resin.
[0023] 33.344g IPDI and 50g DMF were added under stirring at 40°C, and 19.521g HPA and 40g DMF solution were added dropwise into the constant pressure dropping funnel, and the semi-adduct of IPDI / HPA was obtained after 4 hours of reaction.
[0024] According to the remaining -OH content in the hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com