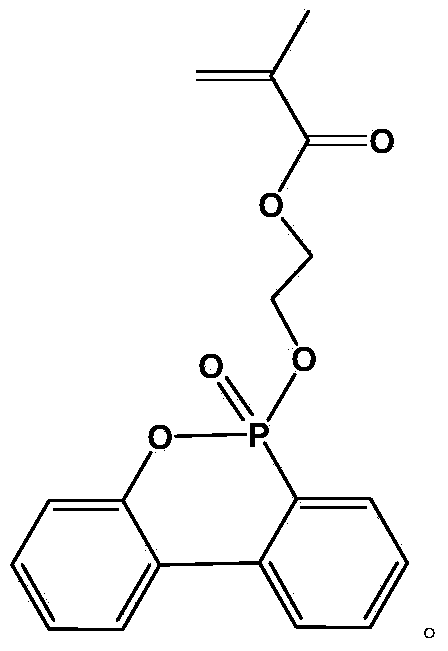
Flame-retardant monomer containing DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) groups and preparation method and application of flame-retardant monomer
A technology of flame retardant monomers and groups, which is applied in the field of flame retardant monomers containing DOPO groups and its preparation, achieving the effects of high yield, easy availability of raw materials, and increased flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
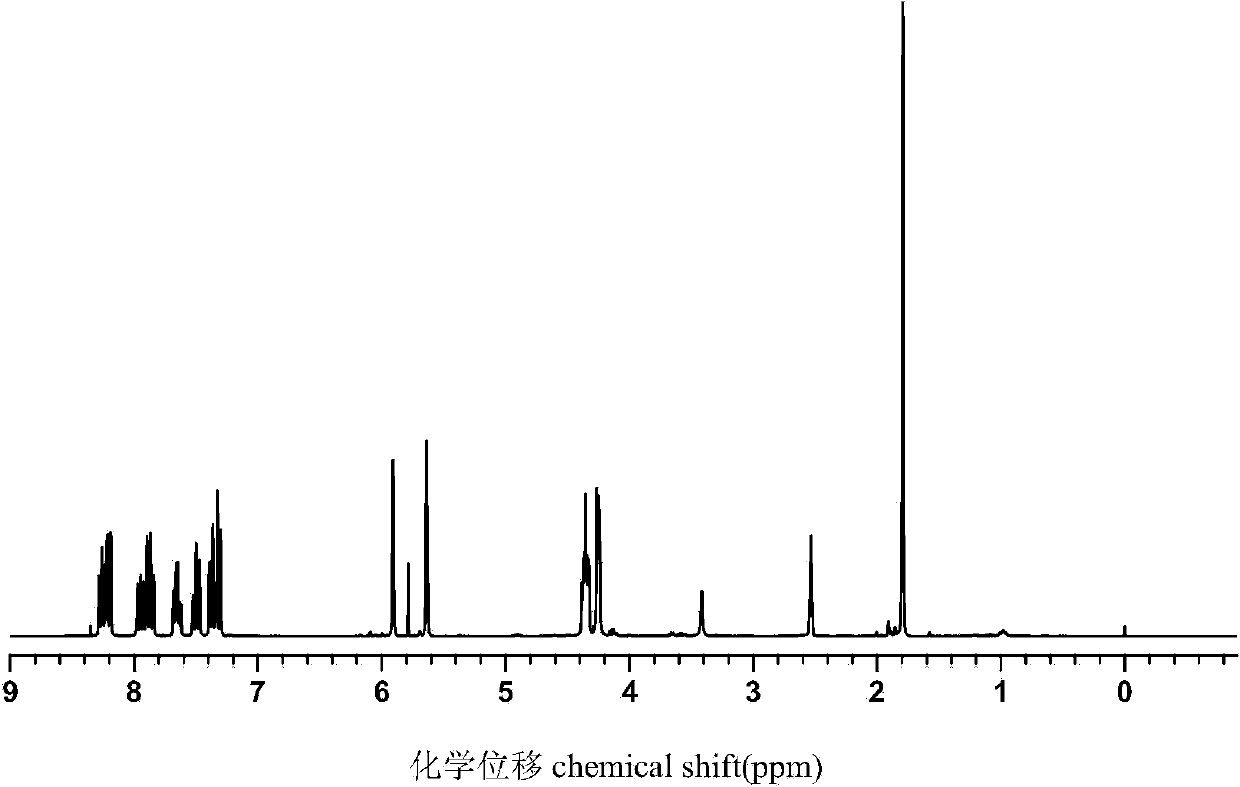
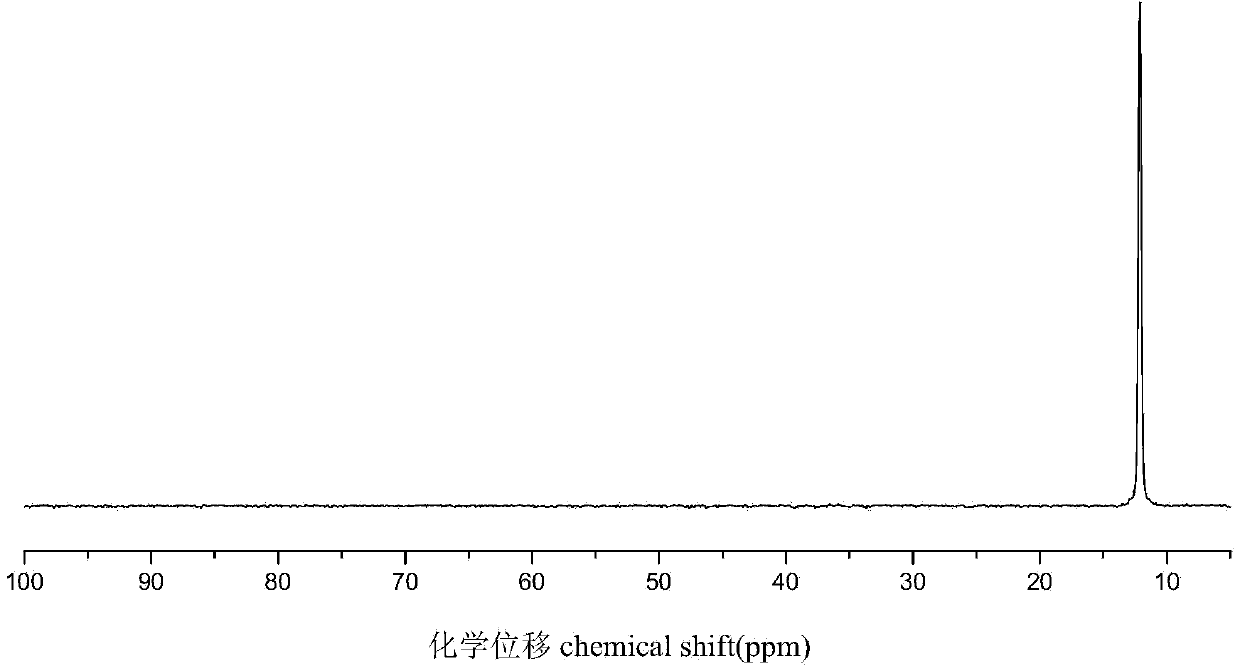
[0029] Dissolve 2.16g (0.01mol) of DOPO in 10mL of dichloromethane, place in an ice-water bath, and add 2mL of carbon tetrachloride under stirring conditions. After 10 min, 2 mL of triethylamine was slowly added. After 1 min, 1.30 g (0.01 mol) of hydroxyethyl methacrylate was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 20° C. and stirring was continued for 8 h to stop the reaction. Afterwards, it was filtered under reduced pressure to remove the triethylamine salt. The filtrate was transferred to a separatory funnel and washed several times with 3% NaOH solution, distilled water, and saturated NaCl aqueous solution. Anhydrous MgSO for organic layer 4 Without drying, filter, evaporate the solvent, and finally concentrate to obtain a yellow oil, which is analyzed by hydrogen spectrum (such as figure 1 ) and phosphorous spectra (such as figure 2 ) was confirmed as the target product flame retardant monomer containing DO...
Embodiment 2
[0031] Dissolve 2.16g (0.01mol) of DOPO in 10mL of tetrahydrofuran, place in an ice-water bath, and add 2mL of carbon tetrachloride under stirring conditions. After 10 min, 2 mL of pyridine was slowly added. After 1 min, 1.95 g (0.015 mol) of hydroxyethyl methacrylate was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 30° C. and stirring was continued for 6 h to stop the reaction. Then vacuum filtration under reduced pressure to remove pyridinium salt. The filtrate was transferred to a separatory funnel and washed several times with 3% NaOH solution, distilled water, and saturated NaCl aqueous solution. Anhydrous MgSO for organic layer 4 Without drying, filter, evaporate the solvent, and finally concentrate to obtain a yellow oil, which is analyzed by hydrogen spectrum (such as figure 1 ) and phosphorous spectra (such as figure 2 ) was confirmed as the target product flame retardant monomer containing DOPO groups, and t...
Embodiment 3
[0033] Dissolve 2.16g (0.01mol) of DOPO in 10mL of toluene, place in an ice-water bath, and add 2mL of carbon tetrachloride under stirring conditions. After 10 min, 2 mL of triethylamine was slowly added. After 1 min, 2.60 g (0.02 mol) of hydroxyethyl methacrylate was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 40° C. and stirring was continued for 4 h to stop the reaction. Afterwards, it was filtered under reduced pressure to remove the triethylamine salt. The filtrate was transferred to a separatory funnel and washed several times with 3% NaOH solution, distilled water, and saturated NaCl aqueous solution. Anhydrous MgSO for organic layer 4 Without drying, filter, evaporate the solvent, and finally concentrate to obtain a yellow oil, which is analyzed by hydrogen spectrum (such as figure 1 ) and phosphorous spectra (such as figure 2 ) was confirmed as the target product flame retardant monomer containing DOPO group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com