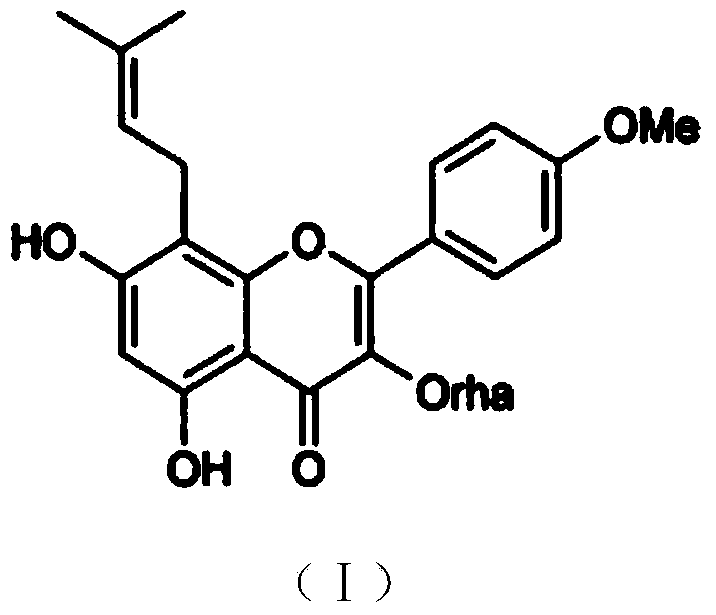
Baohuoside I tablet and preparation method thereof
A technology of baohuoside and tablet, applied in the field of medicine, can solve the problems of complex production process of dry powder inhalation dosage form, poor oral absorption of baohuoside I, unsuitable for large-scale production, etc., so as to increase bioavailability and reduce production. cost, effect of enhancing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
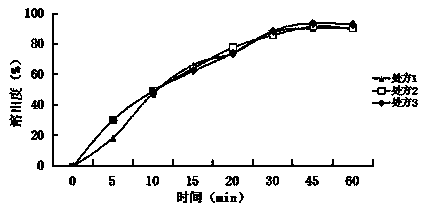
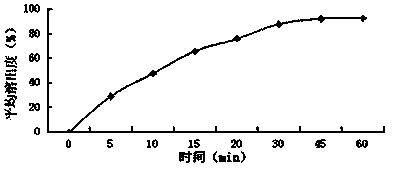
[0037] Embodiment 1: A baohuoside I tablet, which consists of the following raw materials in parts by weight: baohuoside I 6, diluent 55, binder 1.5, disintegrant 1.5, lubricant 0.5, surfactant 0.1;
[0038] The diluent is lactose monohydrate, direct compression lactose, microcrystalline cellulose PH101, microcrystalline cellulose PH102, and the weight ratio is 1:2:2:1; the binder is povidone K30, carboxymethyl Cellulose sodium, and weight ratio is 1:1; Described disintegrant is crospovidone, croscarmellose sodium, and weight ratio is 1:2; Described lubricant is stearic acid, Talc powder, sodium stearyl fumarate, and the weight ratio is 3:1:1; the surfactant is micronized poloxamer 188, micronized poloxamer 407, and the weight ratio is 2:3.
[0039] The preparation method of above-mentioned baohuoside I tablet, it comprises the following steps:
[0040] S1. Weighing: Weigh each raw material according to the above formula, and divide the weighed disintegrant into two parts of ...
Embodiment 2
[0048] Embodiment 2: A baohuoside I tablet, which is composed of the following raw materials in parts by weight: baohuoside I 30, diluent 86, binder 2.5, disintegrant 7, lubricant 5, surfactant 6;
[0049] The diluent is glucose, mannitol, sorbitol, sucrose, cornstarch, and the weight ratio is 1:1:5:3:3; the binder is povidone K90; the disintegrant is cross-linked Povidone, low-substituted hydroxypropyl cellulose, and the weight ratio is 5:3; the lubricant is micronized silica gel, talcum powder, and the weight ratio is 2:1; the surfactant is lauryl sulfate sodium.
[0050] The preparation method of above-mentioned baohuoside I tablet, it comprises the following steps:
[0051]S1. Weighing: Weigh each raw material according to the above formula, and divide the weighed disintegrant into two parts of equal weight, A and B, for later use;
[0052] S2. Mixing: mix the weighed baohuoside I, diluent, disintegrating agent A, and surfactant evenly;
[0053] S3. Pulverization: put p...
Embodiment 3
[0059] Embodiment 3: A baohuoside I tablet, which is composed of the following raw materials in parts by weight: 14 baohuoside I, 74 diluents, 1.7 binders, 4.3 disintegrants, 1.3 lubricants, and 2.9 surfactants;
[0060] The diluent is sorbitol, starch slurry, and the weight ratio is 3:2; the binder is povidone K30, povidone K90, and the weight ratio is 4:3; the disintegrating agent is cross-linked Sodium carboxymethyl cellulose, cross-linked sodium carboxymethyl starch, and the weight ratio is 1.5:1; the lubricant is stearic acid; the surfactant is polyoxyethylene 40 hydrogenated castor oil.
[0061] The preparation method of above-mentioned baohuoside I tablet, it comprises the following steps:
[0062] S1. Weighing: Weigh each raw material according to the above formula, and divide the weighed disintegrant into two parts of equal weight, A and B, for later use;
[0063] S2. Mixing: mix the weighed baohuoside I, diluent, disintegrating agent A, and surfactant evenly;
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com