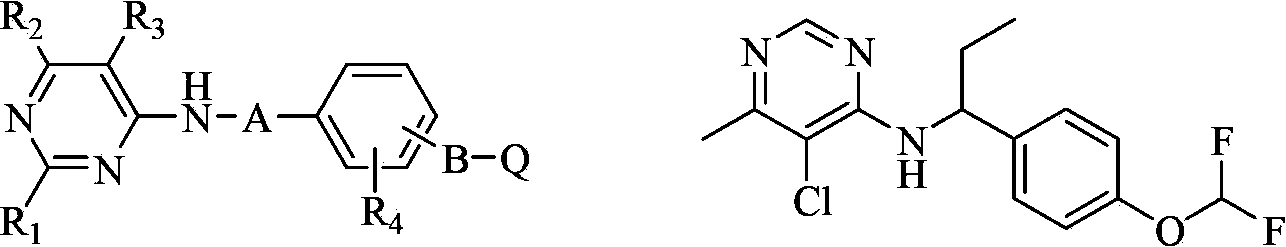
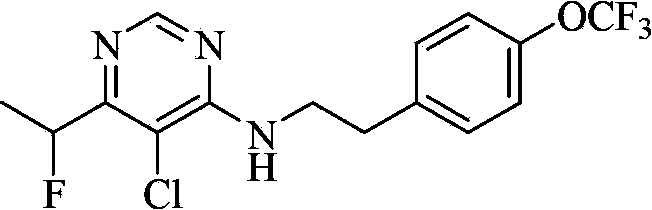
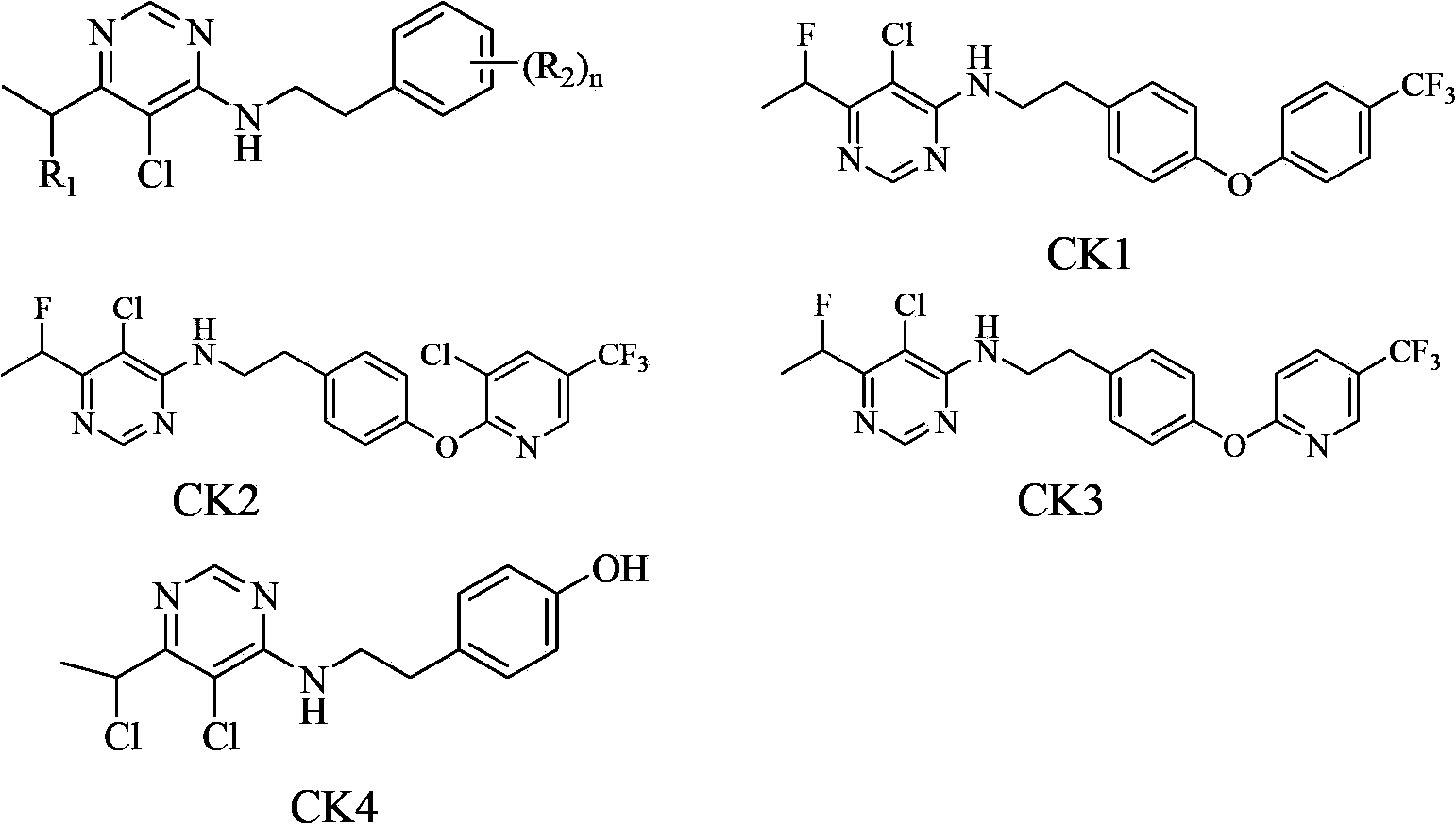
Substituted pyrimidine compound and use thereof
A compound, pyrimidine technology, applied in the field of substituted pyrimidine compounds, can solve the problem of no biological activity report
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] Embodiment 1: the preparation of intermediate 4,5-dichloro-6-methylpyrimidine
[0304] 1) Preparation of 4-hydroxy-5-chloro-6-methylpyrimidine
[0305]
[0306] Slowly add 8.80 g (0.16 mol) of sodium methoxide in methanol solution dropwise to 11.30 g (0.11 mol) of formamidine acetate in 50 ml of methanol solution under stirring at room temperature, and continue stirring at room temperature for 2 h after dropping. Then add 11.17g (0.068mol) intermediate ethyl 2-chloroacetoacetate dropwise to the above solution, and continue to stir the reaction at room temperature for 5-7 hours. After the reaction is monitored by TLC, the solvent is evaporated under reduced pressure, and the pH is adjusted to 5 with hydrochloric acid. ~6, suction filtered to get an orange solid, the aqueous phase was extracted with (3×50ml) ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and precipitated. The residue was dissolved in 50ml of ethyl acetate, left overnight, and filtere...
Embodiment 2
[0310] Embodiment 2: the preparation of intermediate 2-(4-(5-trifluoromethylpyridine-2-oxyl group) phenyl) ethylamine
[0311] 1) Preparation of 2-(4-(5-(trifluoromethyl)pyridine-2-oxyl)phenyl)acetonitrile
[0312]
[0313] Add 18.15g (0.1mol) of 2-chloro-5-trifluoromethylpyridine and 15.96g (0.12mol) of p-hydroxybenzonitrile into 200ml of butanone, add 27.60g (0.2mol) of potassium carbonate, and heat to reflux under stirring , reacted for 4-10 hours, after the reaction was monitored by TLC, the solvent was evaporated under reduced pressure, and 300ml of ethyl acetate was added for extraction. Column chromatography (eluent: ethyl acetate and petroleum ether (boiling range 60-90°C), volume ratio: 1:5) gave 22.50 g of white solid, yield 81%, melting point 48-49°C.
[0314] 2) Preparation of 2-(4-(5-trifluoromethylpyridine-2-oxyl)phenyl)ethylamine
[0315]
[0316] A mixture of 2.78g (0.01mol) 2-(4-(5-trifluoromethylpyridine-2-oxyl group) phenyl) acetonitrile, Raney nicke...
Embodiment 3
[0317] Embodiment 3: Preparation of 4-(2-(5-chloro-6-methylpyrimidine-4-amino)ethyl)phenol
[0318]
[0319] Add 1.13g (0.01mol) 4-hydroxyphenethylamine and 2.02g (0.02mol) triethylamine into 50ml toluene, add 1.63g (0.01mol) 4,5-dichloro-6-methyl dropwise under stirring at room temperature Pyrimidine, continue to react for 4-10 hours. After the reaction is monitored by TLC, the solvent is evaporated under reduced pressure, and (3×50ml) ethyl acetate is added for extraction. The organic phase is washed with 50ml of saturated saline, desolvated under reduced pressure, and the residue is Analysis (eluent: ethyl acetate and petroleum ether (boiling range: 60-90°C), volume ratio: 1:3) gave 2.10 g of white solid, yield 88%, melting point: 177-179°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com