Technetium-99m-labeled higher fatty acid derivative
A higher fatty acid, -99m technology, applied in the field of higher fatty acid derivatives, can solve the problems of poor myocardial uptake, high fat solubility of drugs, affecting myocardial imaging, etc., and achieve the effect of prolonging myocardial retention, reducing blood background, and improving myocardial uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
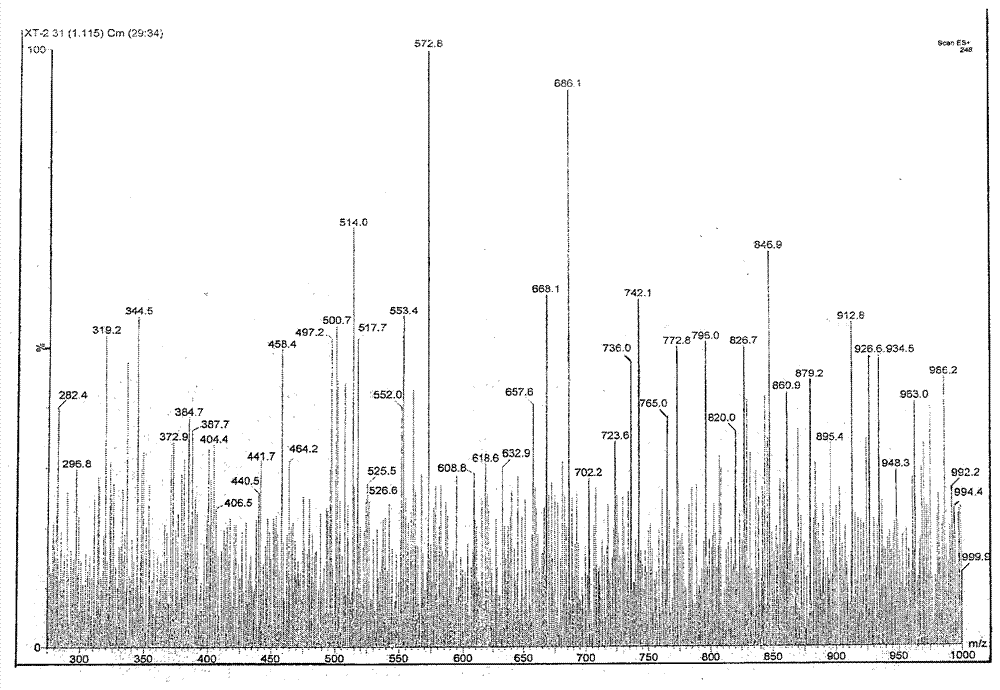
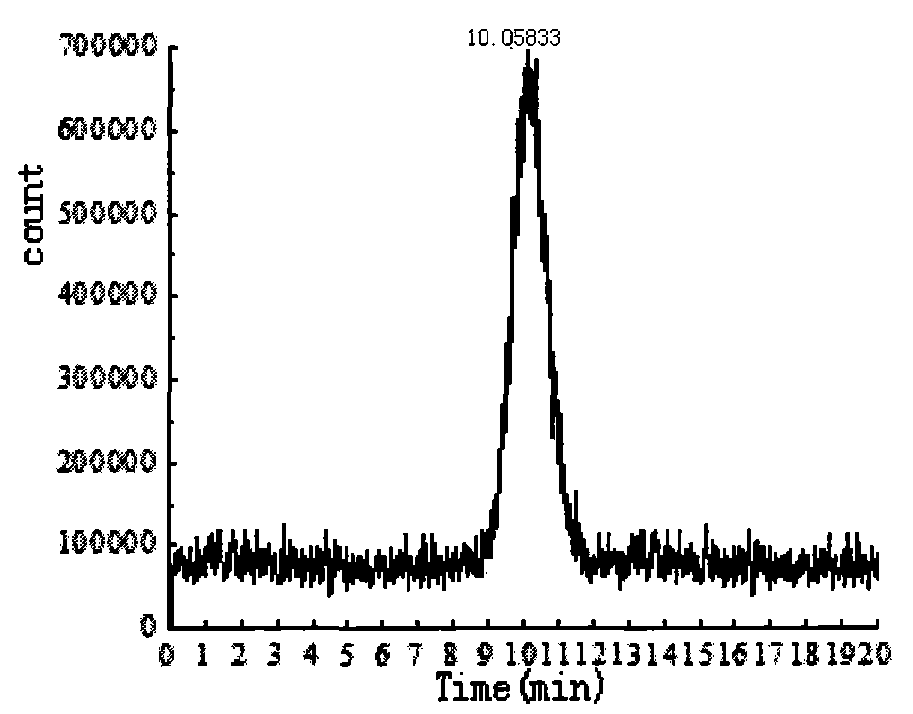
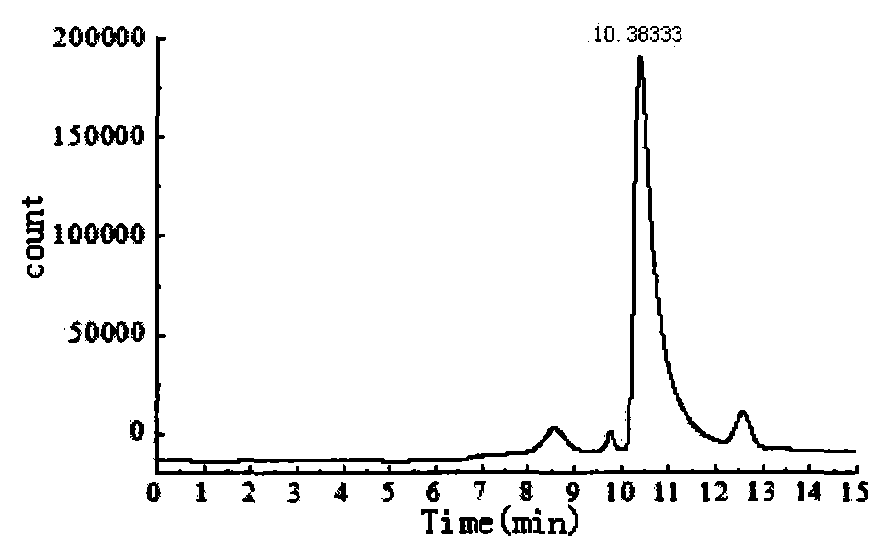
Image
Examples
Embodiment 1
[0030] Example 1: preparation of
[0031] ①Add 30mL of SOCl2 dropwise to 4.06g of dodecanedioic acid (Compound I) in an ice bath, continue to stir for half an hour, and reflux and stir for 12h under an oil bath at 90°C to remove excess SOCl 2 , to obtain a transparent oily product, which is compound II, which is not further purified and is reserved for the next step;
[0032]
[0033] ②Add 5 mL of dry methanol to the compound II obtained in ①, and stir at 60°C for 2 h to obtain a mixture whose main product is compound III, which is left for the next step without further treatment.
[0034]
[0035] ③Add 20mL of SOCl to 3g of thienylpropionic acid 2 , and stirred for 2h, 2.9g of thienylpropionyl chloride was obtained with a yield of 96%.
[0036]
[0037] 4. under ice-bath conditions, the thiophene propionyl chloride that step 3. gained is joined in the dichloromethane that is dissolved with 2.58g ferrocene, stirs 4 hours under room temperature, obtains the mixed s...
Embodiment 2
[0043] Example 2: preparation of
[0044] ① Dissolve 30mL of SOCl in an ice bath 2 Add dropwise to 4.06 g of compound I, continue to stir for 30 min, and then reflux and stir for 12 h under the condition of an oil bath at 90 ° C to remove excess SOCl 2 , to obtain a transparent oily product, that is, compound II, which was reserved for the next step without further purification.
[0045]
[0046] ② Add 5 mL of dry methanol to ①, stir at 60°C for 2 h to obtain a mixed solution of compound III, which will be used for the next step without further treatment.
[0047]
[0048] ③Add 20mL of SOCl to 3g of thienylpropionic acid 2 , and stirred for 2 hours to obtain thienylpropionyl chloride, which was not treated and was reserved for the next step.
[0049]
[0050] 4. under ice-bath conditions, the thiophene propionyl chloride that step 3. gained is joined in the dichloromethane that is dissolved with 2.58g ferrocene, stirs 4 hours under room temperature, obtains the m...
Embodiment 3
[0056] Example 3: preparation of
[0057] ① Dissolve ethyl 5-bromopentanoate (3.8mL, 24.0mmol) and thiourea (2.7g, 34mmol) in 50mL of ethanol, reflux for 20h, remove the solvent under reduced pressure, add 7.5M NaOH aqueous solution (aq) (50mL , 360mmol), the mixture was stirred at 90°C under nitrogen protection for 16h; in ice bath, 2M H 2 SO 4 aq, adjust the pH to 1, the product with CH 2 Cl 2 Extracted twice with anhydrous MgSO 4 After drying, filtering, and removing the solvent, the product was directly used in the next reaction without further treatment.
[0058]
[0059] ② Dissolve ethyl 5-bromopentanoate (3.8mL, 24.0mmol) in 16.7M (8mL) NaOH, add it dropwise to the NaOH solution of compound III under ice-bath conditions, and then Under the condition of stirring for 24h; the reactant was adjusted to pH 1 with concentrated HCl, and CH 2 Cl 2 After extracting 5 times, the organic layers were combined, dried with anhydrous NaSO4, filtered, and the solvent was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com