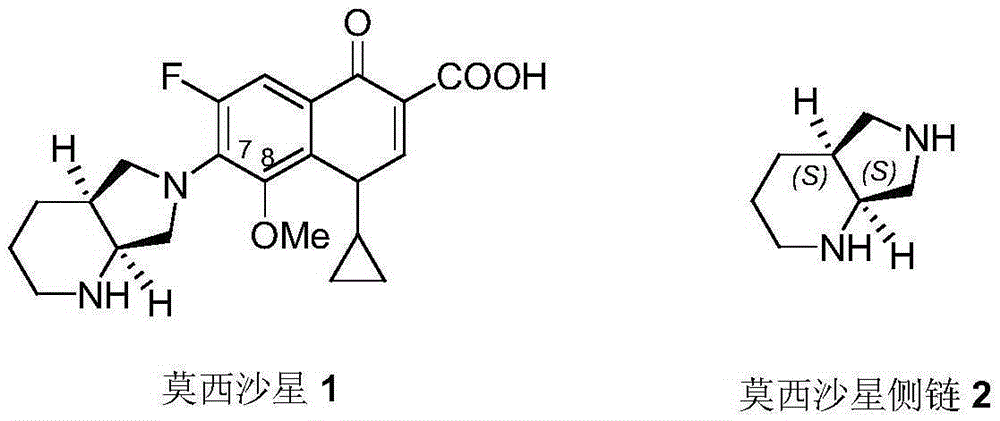
A kind of preparation method and application of PD catalyst with nanoporous structure
A nanoporous, catalyst technology, applied in the field of medicine, can solve the problems of easy desorption, reduced catalytic activity, poor effect, etc., and achieve the effect of easy recovery and improved catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 0.5 g azobisisobutyronitrile, 2.5 ml divinylbenzene, 1.5 g 1,3,5-triacryloylhexahydro-1,3,5-triazine, 0.01 g ferric chloride, 0.025 g persulfate Add ammonium co-initiator to 80 ml tetrahydrofuran in turn, add 3 ml water as a co-solvent, stir at room temperature for 3 hours (stirring rate is about 800rpm), reflux at 100°C for 12 hours, further solvent thermal crystallization, 120°C reactor treatment After 24 hours, take out the reaction kettle, open the cover to evaporate the solvent (humidity is about 60%, evaporate for 24 hours) to obtain the crude product. The residual salt in the sample was removed by washing with water, and vacuum-dried at 80° C. to obtain a nanoporous material with open pores.
[0035] Weigh 2g nanoporous material PDVB-THAT and 0.224gPd(OAc) 2 In 30mL tetrahydrofuran and 10mL water, after stirring at room temperature for 3 hours, add 68mgNaBH 4 to Pd(OAc) 2 to restore. After the reactant was stirred at room temperature for 10 hours, the catalys...
Embodiment 2
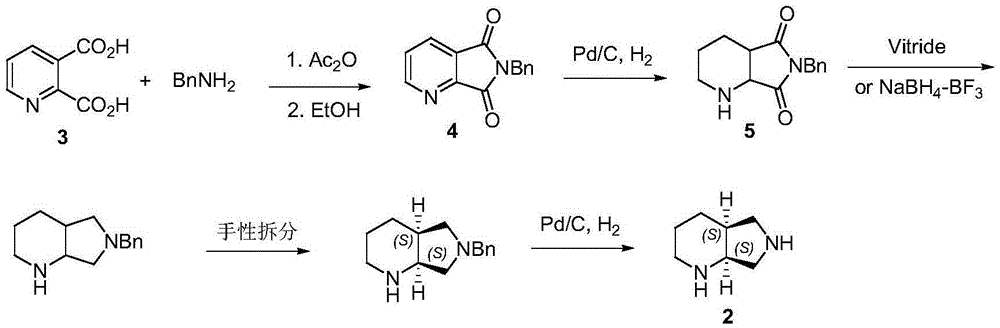
[0037] Catalytic hydrogenation of 6-benzyl-5,7-dioxo-pyrrolo[3,4,b]pyridine: add 10g of 6-benzyl-5,7-dioxo-pyrrolo[3, 4,b] pyridine and 100 mL n-butyl acetate, add 1 g PDVB-THATPd 0 (the content of Pd is 5%), the pressure of flushing into hydrogen is 5bar, heating at 50g for 7 hours, filtering, and recovering PDVB-THATPd 0 Catalyst, the filtrate obtains 9.8g light yellow solid after removing the solvent, it is determined as racemic cis-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4 ,3,0] nonane. The yield was 97%.
[0038]
[0039] 1H-NMR (CDCl 3 ,400MHz)δppm7.32–7.24(m,5H),4.64(s,2H),3.84(d,1H,J=6.8Hz),2.89-2.2.83(m,1H),2.82-2.76(m, 1H),2.70-2.64(m,1H),2.08(brs,1H),2.03-1.93(m,1H),1.68–1.61(m,1H),1.54-1.50(m,1H).
Embodiment 3
[0041] The catalyst is reused for the second time: add 10g of 6-benzyl-5,7-dioxo-pyrrolo[3,4,b]pyridine and 100mL of n-butyl acetate in the autoclave, add the catalyst recovered in Example 2 PDVB-THATPd 0 Afterwards, the pressure of flushing hydrogen is 5bar, heated at 50°C for 7 hours, filtered, and recovered PDVB-THATPd 0 Catalyst, the filtrate obtained 9.7g light yellow solid after removing the solvent, and was determined to be racemic cis-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4 ,3,0] nonane. The yield is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com