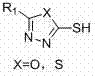Derivatives containing thiadiazole or oxadiazole and application of derivatives in prevention and control of agricultural plant diseases
A technology of thiadiazole derivatives and derivatives, applied in the direction of chemicals, applications, and biocides for biological control, can solve the problems of unreported agricultural activity, achieve simple structure, broad application prospects, and production low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
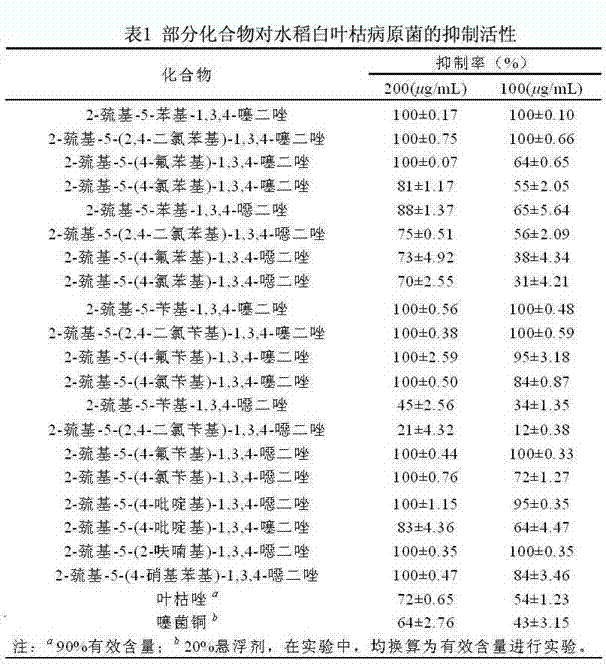
[0044] Example 1: Determination of Indoor Inhibition of Bacterial Disease Pathogen Activity of Some Compounds
[0045] (1) Determination of the indoor activity of some compounds against rice bacterial blight
[0046] The rice bacterial blight pathogen was incubated in M210 (enzymatically hydrolyzed casein: 8 g, sucrose: 5 g, yeast extract: 4 g, K 2 HPO 4 : 3 g, MgSO 4 ·7H 2O: 0.3 g, agar: 15 g, secondary water: 1 L, pH = 7.0) Streak on the solid medium, culture at 28 °C until a single colony grows. Pick a single colony of rice bacterial blight pathogen on M210 solid medium to M210 liquid medium (enzymatically hydrolyzed casein: 8 g, sucrose: 5 g, yeast extract: 4 g, K 2 HPO 4 : 3 g, MgSO 4 ·7H 2 O: 0.3 g, secondary water: 1 L, pH=7.0), cultured on a constant temperature shaker at 28 °C and 180 rpm until the logarithmic phase of growth was set aside.
[0047] The synthesized compound and the control drug were respectively configured at a concentration of 200 and 100 mu...
Embodiment 2
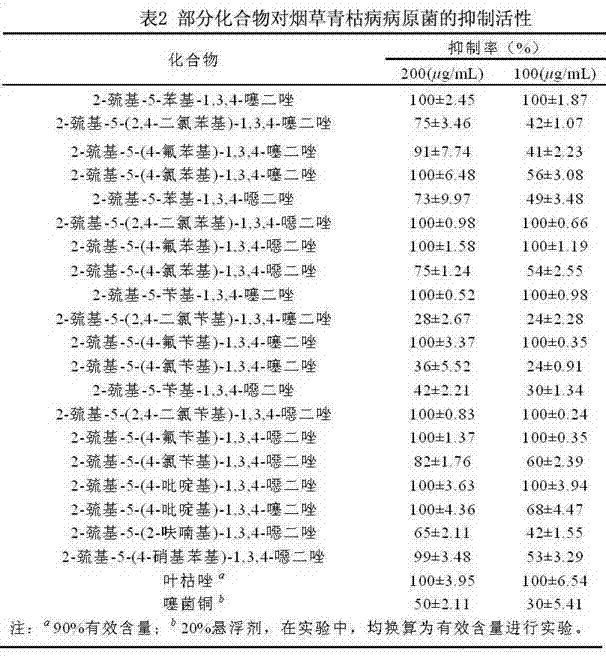
[0061] Embodiment 2: Determination of the activity of some compounds inhibiting fungal pathogens of crops indoors
[0062] The antibacterial activity of the compounds was determined by the in vitro growth rate method. Heat the potato dextrose agar medium (PDA medium: 200 g potato, 20 g agar, 20 g glucose, 1000 mL distilled water) to the molten state (40-60 ℃), and dissolve 10 mL of the drug solution (10 times the final concentration of the drug solution ) into 90 mL PDA medium, shake well, pour evenly into a 9 cm diameter petri dish, place it horizontally, and wait for cooling to solidify. At the edge of the fresh pathogenic bacteria colony that has been cultured for 4 days, a 4 mm diameter bacterial disk was punched out with a puncher, and the bacterial disk was placed upside down in the center of the PDA plate containing the drug, and then placed in a constant temperature and humidity incubator at 27 °C for upside-down cultivation. When the colonies of the blank control gre...
Embodiment 3
[0067] Embodiment three: highly active compound toxicity regression equation and inhibitory medium concentration (EC 50 ) value determination
[0068] (1) Regression equation and EC of compound toxicity to rice bacterial blight pathogen 50 Determination of value
[0069] The synthetic compound and the control agent were respectively prepared into 5 corresponding concentrations of toxic M210 liquid medium, 5 mL was taken in a test tube, and the OD value of the toxic sterile liquid medium was measured with a microplate reader (OD 595 ), adding 40 mu L containing the M210 liquid medium containing the pathogenic bacteria of Xanthomonas oryzae, then cultured on a constant temperature shaker at 28 °C and 180 rpm for 24-48 h, and measured the OD value of each concentration of the bacterial solution with a microplate reader (OD 595 ). In addition, the OD value of the toxic and sterile M210 liquid medium containing the control drug and the OD value of the bacterial solution of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com