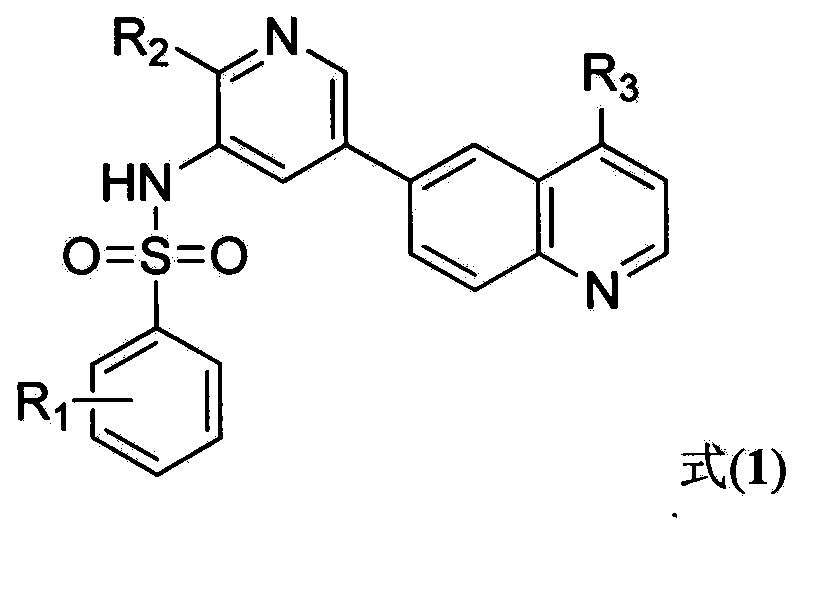
N-(5-(quinolyl-6-yl) pyridyl-3-yl)benzsulfamide derivatives, and preparation method and treatment use thereof
A technology of difluorobenzenesulfonamide and methoxypyridine, which is applied in the field of medicine and can solve the problems of low selectivity and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
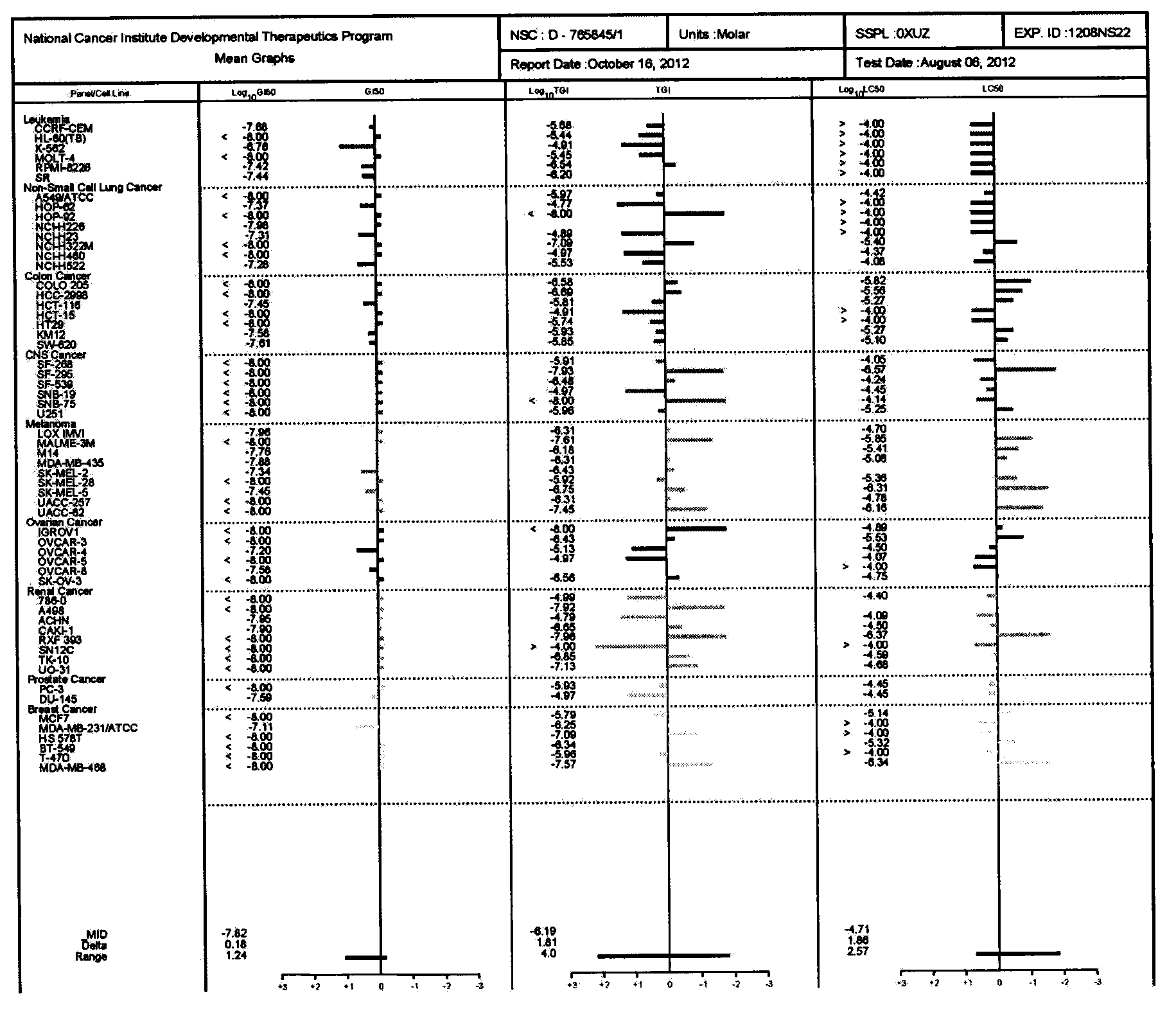
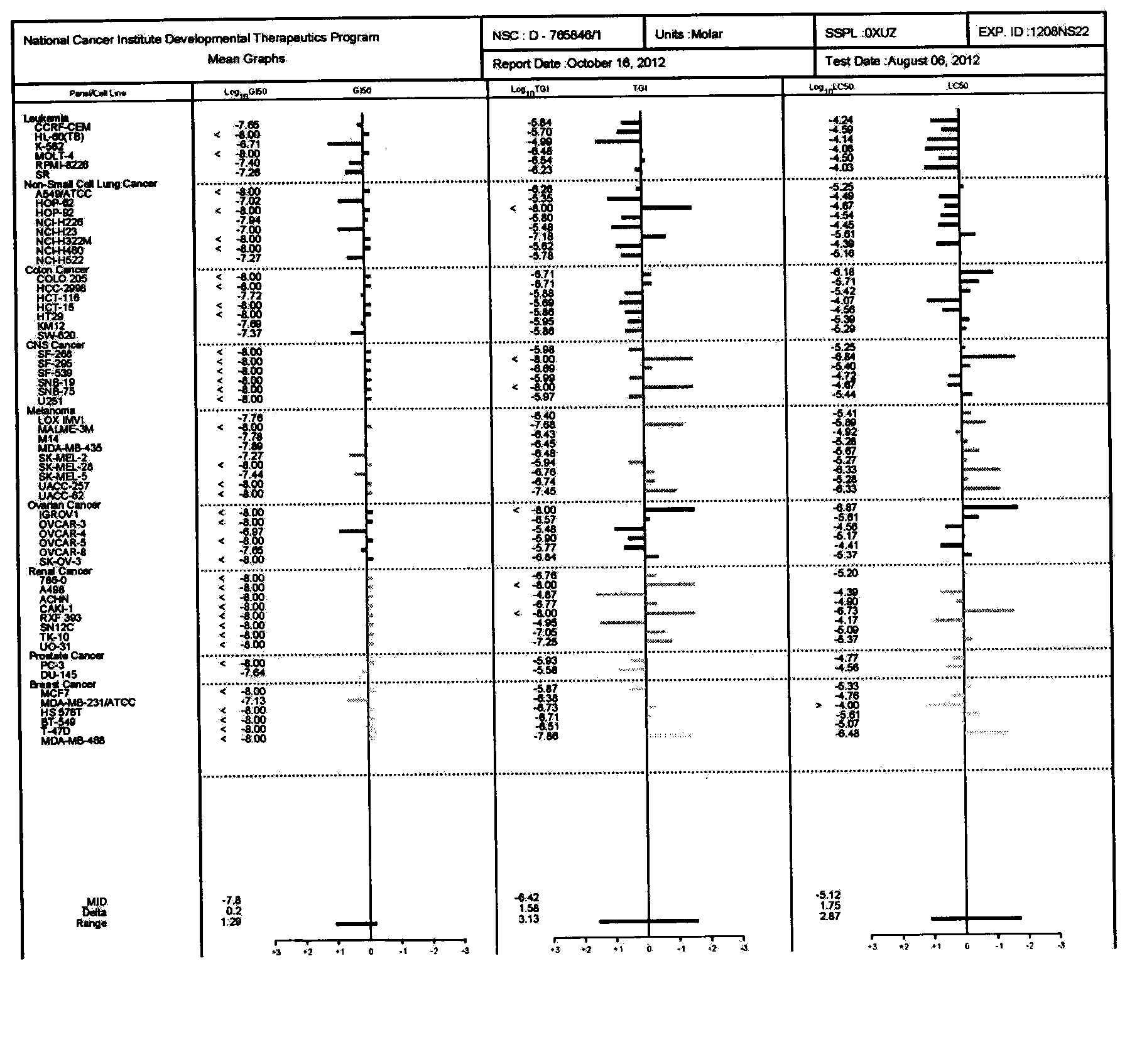
Image
Examples
Embodiment 1
[0128] N-(5-(4-(1H-1,2,4-triazol-1-yl)quinolin-6-yl)-2-methoxypyridin-3-yl)-2,4-di The preparation of fluorobenzenesulfonamide (IV, compound number 1 in the table):
[0129]
[0130] a) Preparation of 6-bromo-4-(1H-1,2,4-triazol-1-yl)quinoline (III):
[0131] Add 6-bromo-4-chloroquinoline (0.5g, 2.06mmol) and triazole (0.71g, 10.30mmol) into a 25ml single-necked bottle, dissolve in 4mL of 1,4-dioxane, and reflux at 90°C Stir and heat for 4 hours, cool to room temperature, add chloroform and saturated sodium bicarbonate solution for extraction, wash the organic layer with water and saturated brine respectively, dry over anhydrous magnesium sulfate, and evaporate to dryness under reduced pressure, 0.24 g of the solid product crystallized with ether, yield rate of 42.9%. MS (ESI) m / z: 275.16 (M+H+).
[0132] B) N-(5-(4-(1H-1,2,4-triazol-1-yl)quinolin-6-yl)-2-methoxypyridin-3-yl)-2,4 - Preparation of difluorobenzenesulfonamide (IV, compound number 1 in the table):
[0133]...
Embodiment 2-3
[0135] Compounds 2 and 3 in Table 1 can be prepared from corresponding raw materials by referring to the general method for preparing the compound of Example 1.
Embodiment 4
[0137] N-(5-(4-chloroquinolin-6-yl)-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide (IV, compound number 4 in the table)
[0138]
[0139] a) Preparation of 4-chloro-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline (HI):
[0140] Add raw material (II) (0.50g, 2.06mmol) in the 50ml eggplant-shaped bottle, dissolve with 13mL of dioxane, add biboronic acid pinacol ester (0.79g, 3.10mmol) and potassium acetate (0.81g , 8.25mmol) and palladium catalyst PdCl 2 (dppf)CH 2 Cl 2 (0.128g, 0.157mmol), heated at 90°C for 3 hours under nitrogen protection. After cooling to room temperature, the solvent was evaporated to dryness, and the resulting mixture was extracted with ethyl acetate (20 mL) and water (20 mL). The aqueous layer was extracted with a small amount of ethyl acetate (15mL×2), the organic layers were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 1.15g of crude product (PH=6), which was purified by silica gel column chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com