Electrophilic fluoroform sulfenyl reagent and synthetic method and application thereof
A technology of trifluoromethylthio group and synthesis method, which is applied in sulfide preparation, organic chemistry and other directions, can solve the problems of low substrate tolerance, high reagent toxicity, difficult operation and the like, and achieves simple method, easy synthesis and operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
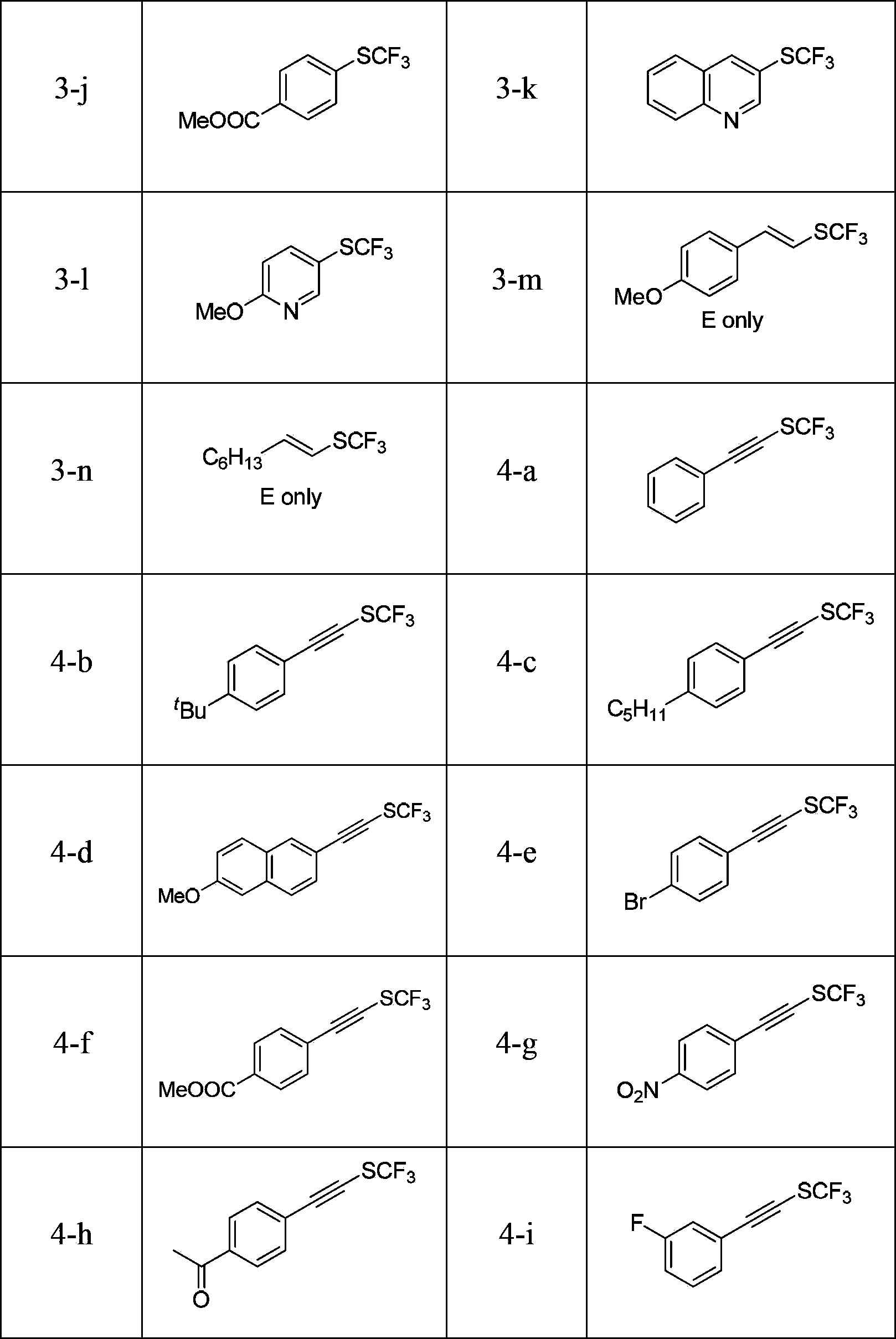
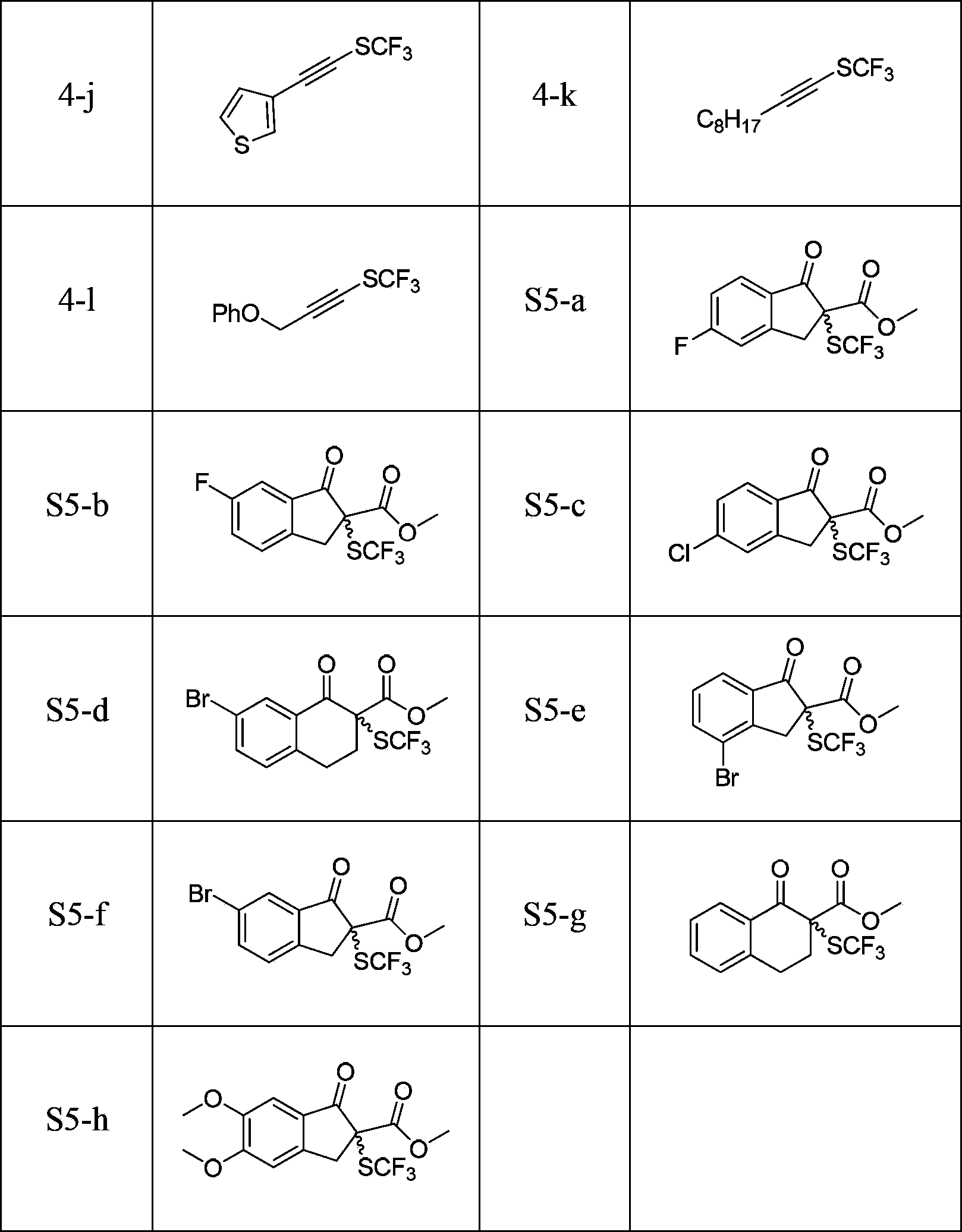
[0027] Example 1 Synthesis of Electrophilic Trifluoromethylthiolation Reagent
[0028] (1) Add 0.208g (10mmol) AgSCF to an oven-dried 100ml Schlenk tube equipped with a stirrer in an argon atmosphere. 3and 0.296 g (10 mmol) of starting material P-152, and 50 ml of freshly purified tetrahydrofuran was added. After preheating at 50°C, the reaction was stirred at this temperature for 1 hour, and AgSCF was detected by fluorine spectroscopy. 3 Completely disappeared, filtered through celite and concentrated to minimum volume in vacuo. The products were separated by flash column chromatography (wet loading). Eluent: 30-60°C petroleum ether, Rf: 0.95. Yield: 30%. The product was further purified using Kugelrohr distillation.
[0029] 1 H NMR (300MHz, CDCl 3 ,293K,TMS)δ8.05(d,J=7.8Hz,1H),7.42-7.32(m,1H),6.97(t,J=7.2Hz,1H),1.86(s,6H)ppm; 19 F NMR (375MHz, CDCl 3 )δ-51.2(s,3F)ppm;IR(KBr):ν=3057,2976,2930,1583,1560,1464,1428,1384,1366,1262,1230,1174,1087,1048,10104,952,904,855,7...
Embodiment 2
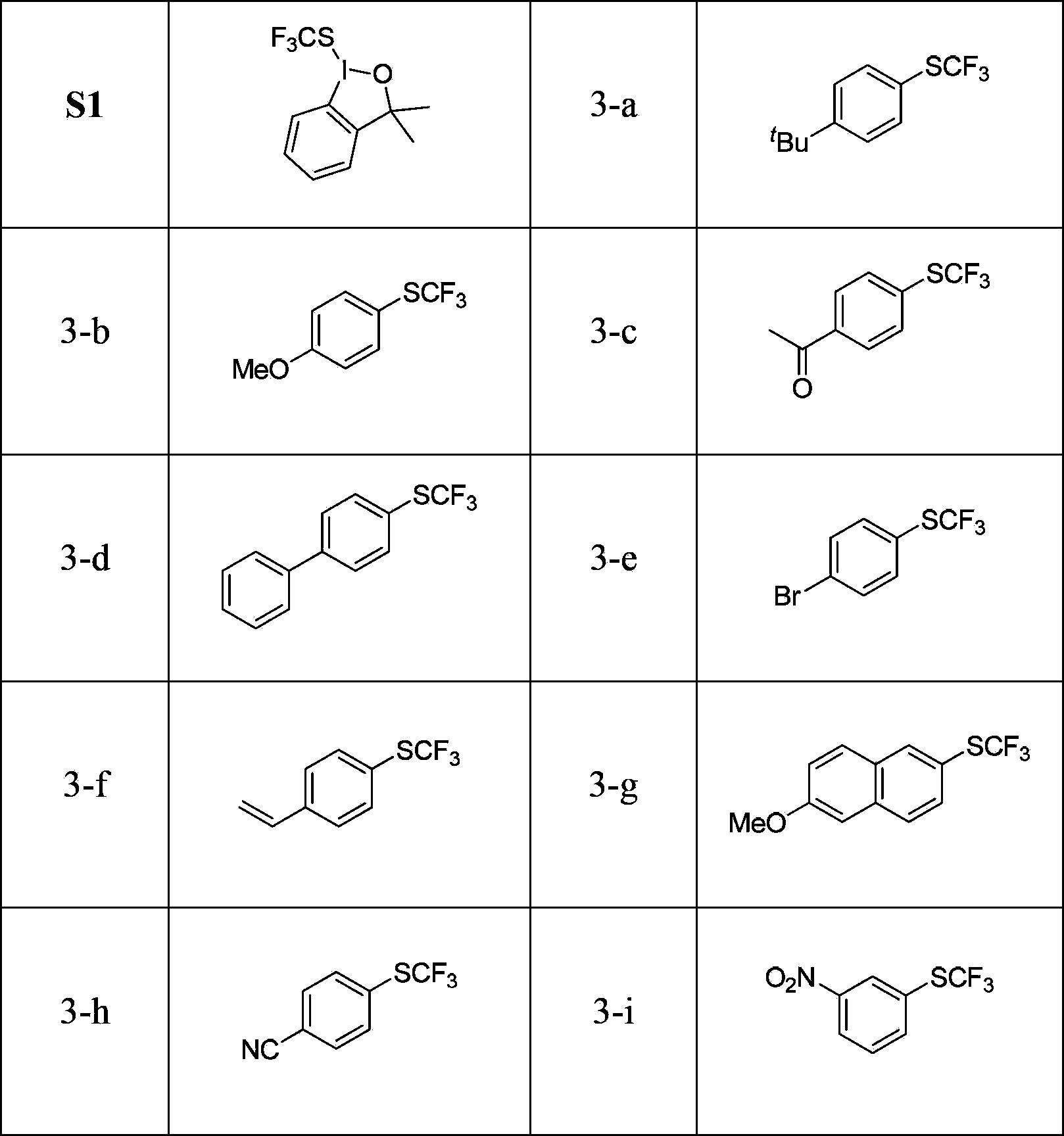
[0040] Example 2 Application of electrophilic trifluoromethylthio reagent
[0041] The electrophilic trifluoromethylthio reagent is S1.
[0042] p-tert-butylphenyl trifluoromethyl sulfide
[0043] 1-tert-Butyl-4-[(trifluoromethyl)thio]benzene 3-a
[0044] To an oven-dried sealed tube equipped with a stirring bar was sequentially added 0.0185 g (0.05 mmol) Cu(MeCN) under an argon atmosphere 4 PF 6 , 0.0160g (0.1mmol) 2,2'-bipyridine, 0.138g (1.0mmol) anhydrous potassium carbonate, 0.3620g (0.5mmol) electrophilic trifluoromethylthio reagent and 0.1157g (0.65mmol) 4-tertiary butylphenylboronic acid, and 2.5 ml of freshly purified diethylene glycol dimethyl ether was added. The reaction was stirred at 35°C and detected by fluorine spectrum until the electrophile completely disappeared, which generally took 15 hours. 25ml of distilled water and 20ml of anhydrous ether were added to the reaction system for extraction, the organic phase was separated, the aqueous phase was extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com