2-(4-bromophenyl) quinoline-4-formic acid complex and preparation method and application thereof
A bromophenyl complex technology, applied in the field of 2-(4-bromophenyl) quinoline-4-carboxylic acid complex and its preparation and application, can solve the problems of strong irritation, high toxicity, difficult absorption, etc. , to achieve good stability, improved antibacterial performance, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
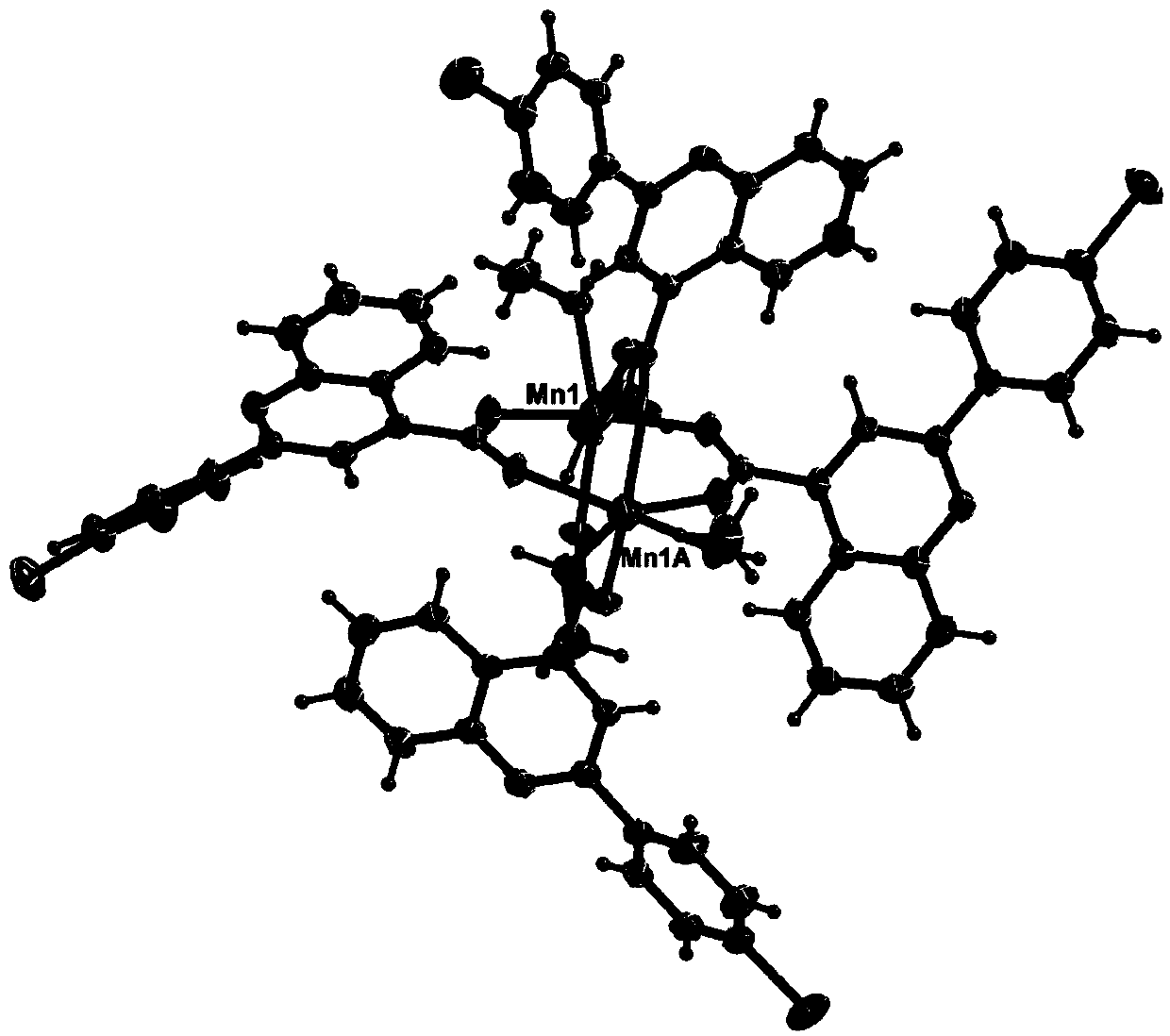
[0031] 2-(4-Bromophenyl)quinoline-4-carboxylic acid manganese(Ⅱ)
[0032] (1) Dissolve isatin (1.18g, 8.00mmol), 4-bromoacetophenone (0.40g, 2.00mmol), potassium hydroxide (2.24g, 40.00mmol) in a mixed solution of 2mL ethanol and 18mL water, Heat to reflux for 12 hours, after cooling, pour the reaction solution into 20mL of water, adjust the pH of the system to 4 with 1M hydrochloric acid, filter the resulting precipitate, wash with water to obtain a yellow solid 2-(4-bromophenyl)quinoline-4-carboxylic acid 597mg, yield: 91%;
[0033] Detect 2-(4-bromophenyl) quinoline-4-carboxylic acid to get IR(KBr, cm -1 ):3445,2361,1714,1588,1544,1490,1403,1367,1227,1197,1075,1007,831,802,766,713,541,514. 1 H NMR(500MHz,DMSO,δ):8.65(d,J=8.0Hz,1H),8.46(s,1H),8.27(d,J=8.5Hz,2H),8.17(d,J=8.0Hz, 1H),7.87(t,1H),7.77(d,J=8.5Hz,2H),7.21(t,1H).Anal.Calcd for C 16 h 10 BrNO 2:C,58.56;H,3.07;N,4.27.Found:C,58.67;H,3.04;N,4.29%;
[0034] (2) Dissolve 2-(4-bromophenyl)quinoline-4-carboxylic aci...
Embodiment 2
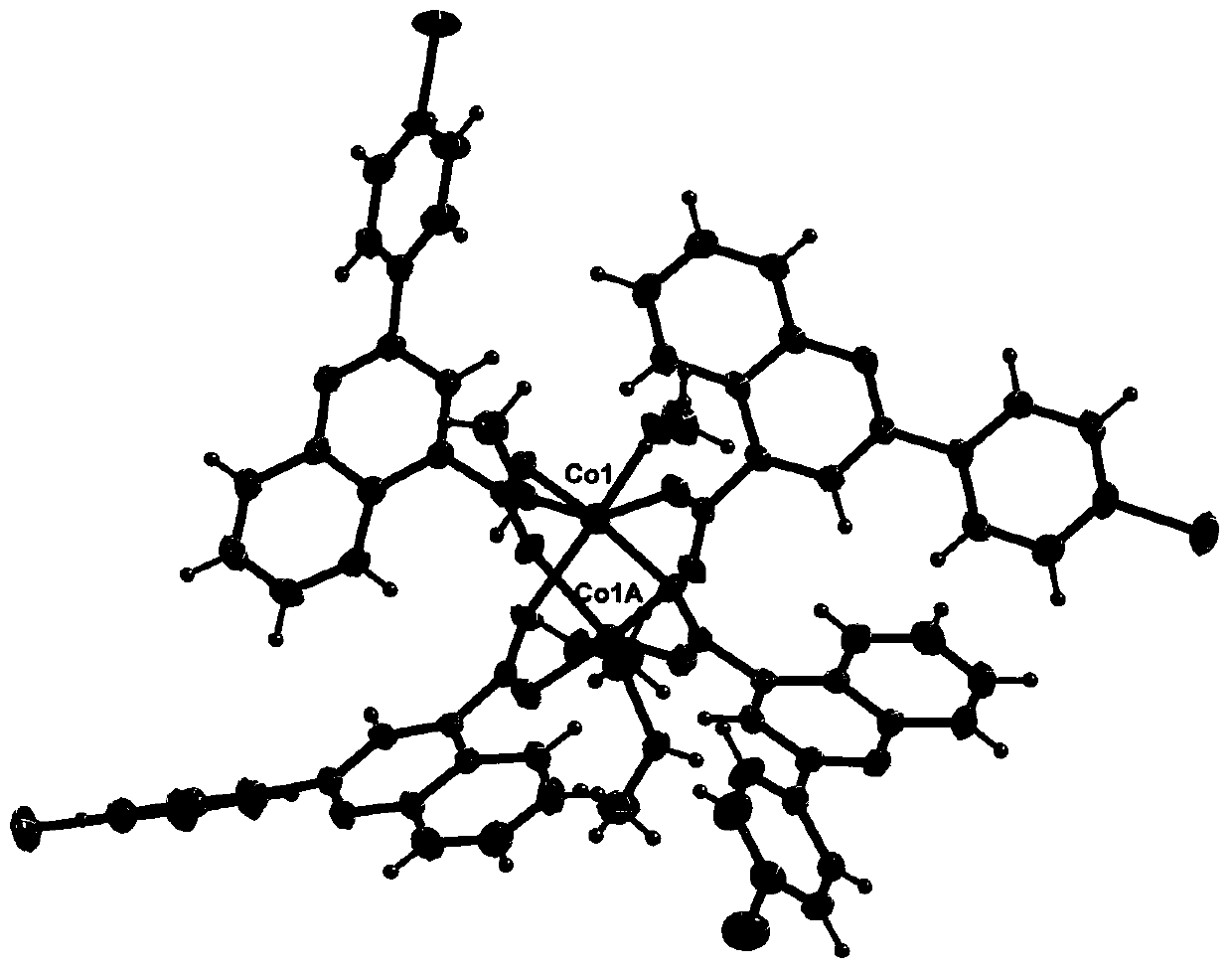
[0039] 2-(4-Bromophenyl)quinoline-4-carboxylate cobalt(Ⅱ)
[0040] Step (1) is the same as step (1) in Example 1;
[0041] Step (2) Dissolve 2-(4-bromophenyl)quinoline-4-carboxylic acid (13.1mg, 0.04mmol) in 6mL of methanol, add cobalt acetate (10.0mg, 0.04mmol) in 4mL of methanol solution, Stir at room temperature for 4 minutes, filter, and stand for 4 days, 6.0-8.0 mg of yellow crystals appear, filter, wash with methanol three times, and dry in an anhydrous calcium chloride desiccator to obtain the product. Yield 45%.
[0042] The result of elemental analysis of the product: Calculated value: C(%), 52.53; H(%), 3.37; N(%), 3.60;
[0043] Measured value: C(%), 52.69; H(%), 3.35; N(%), 3.62
[0044] IR(KBr,cm -1 ):3446,3065,2361,1645,1603,1536,1464,1404,1367,1032,810,759,666,468.
Embodiment 3
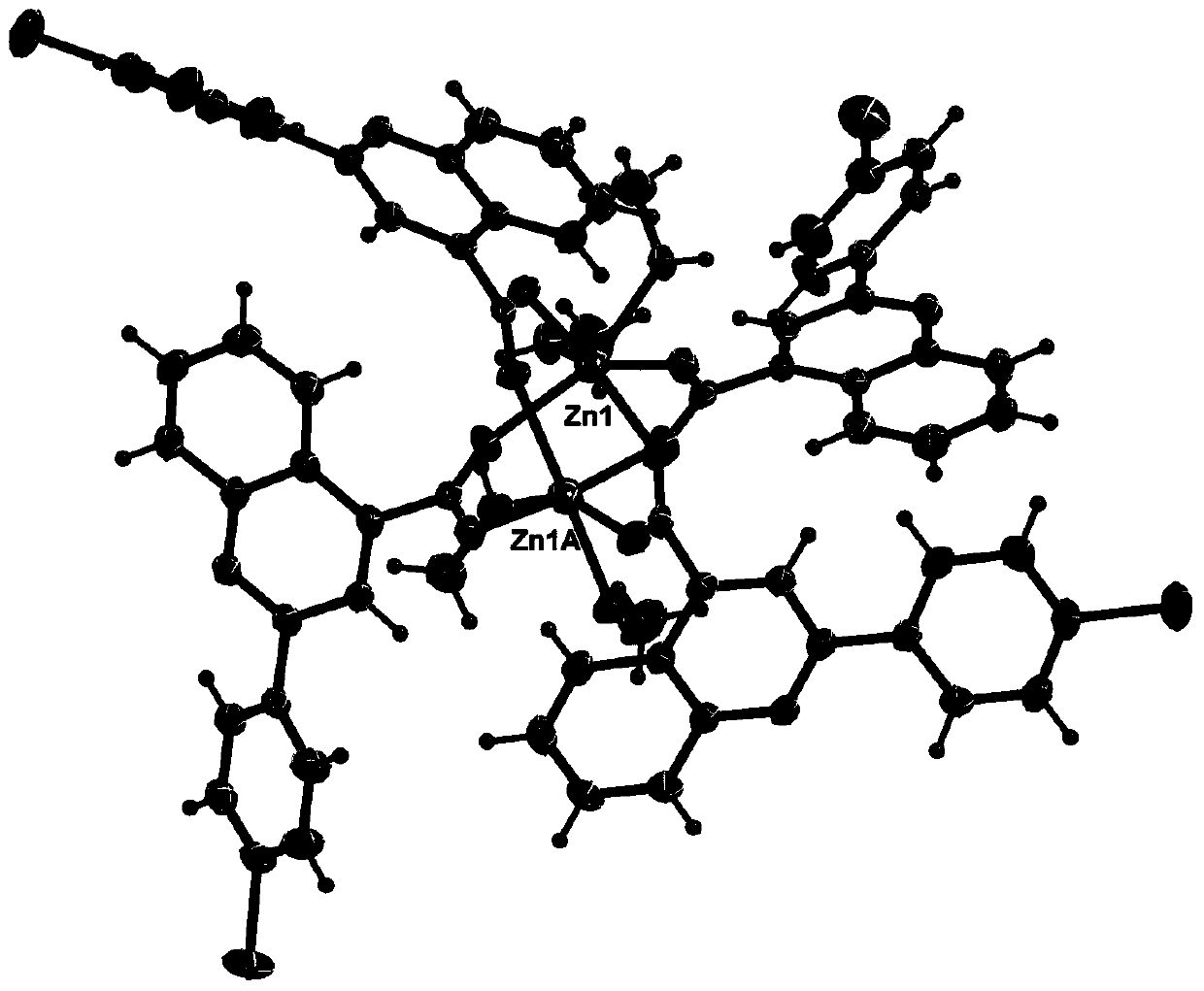
[0046] 2-(4-Bromophenyl)quinoline-4-carboxylate zinc(Ⅱ)
[0047] Step (1) is the same as step (1) in Example 1;
[0048] Step (2) 2-(4-bromophenyl)quinoline-4-carboxylic acid (13.1mg, 0.04mmol) was dissolved in 8mL of methanol, and added to a solution of zinc acetate (8.8mg, 0.04mmol) in 4mL of methanol, Stir at room temperature for 3 minutes, filter, and stand for 3 days, 11.0-12.0 mg of yellow crystals appear, filter, wash with methanol three times, and dry in an anhydrous calcium chloride desiccator to obtain the product. Yield 72%.
[0049] The result of elemental analysis of the product: Calculated value: C(%), 52.10; H(%), 3.34; N(%), 3.57;
[0050] Measured value: C(%), 52.31; H(%), 3.32; N(%), 3.58;
[0051] IR(KBr,cm -1 ):3445,3063,2361,1651,1588,1547,1403,1321,1106,1073,1029,810,761,665,469.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com