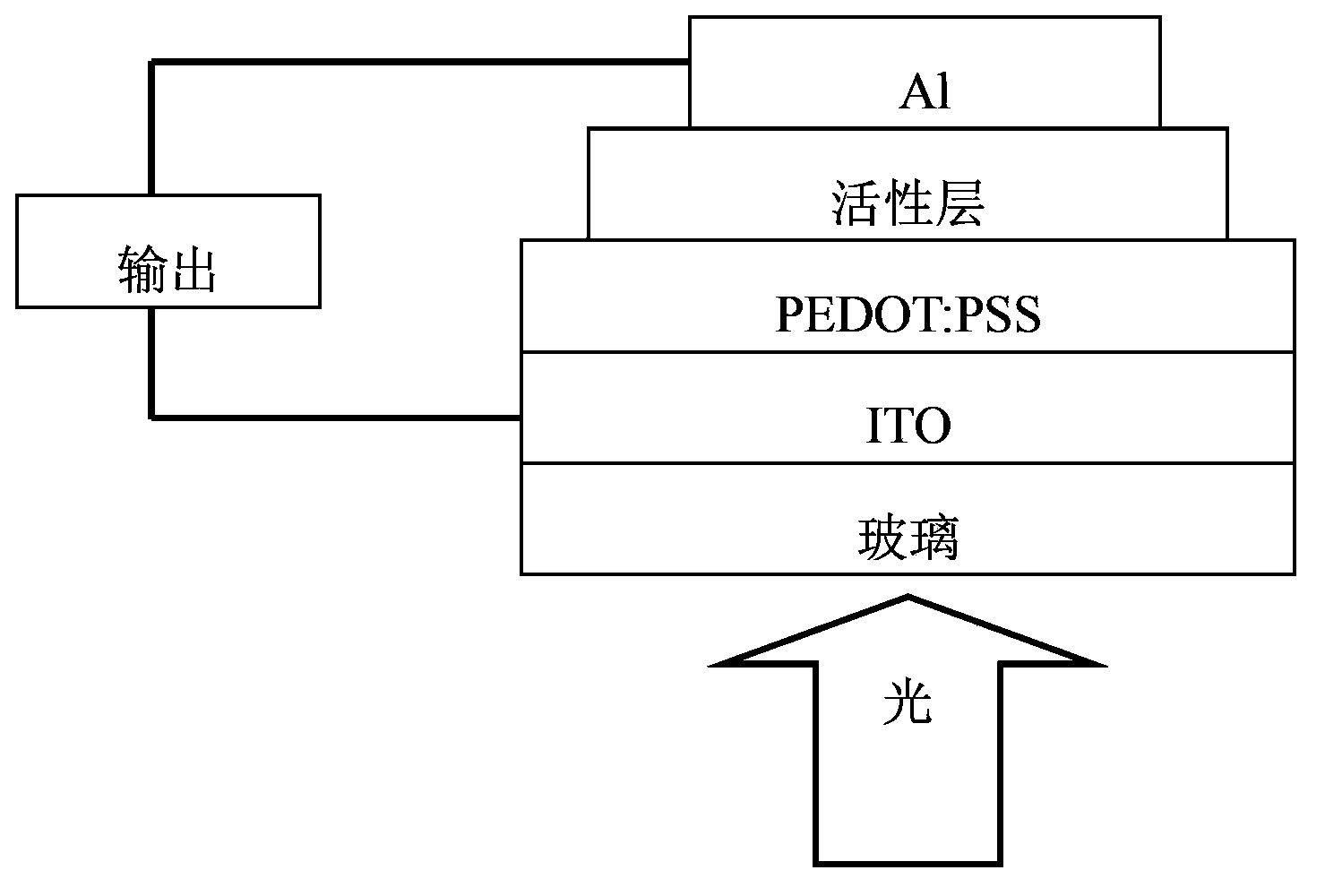
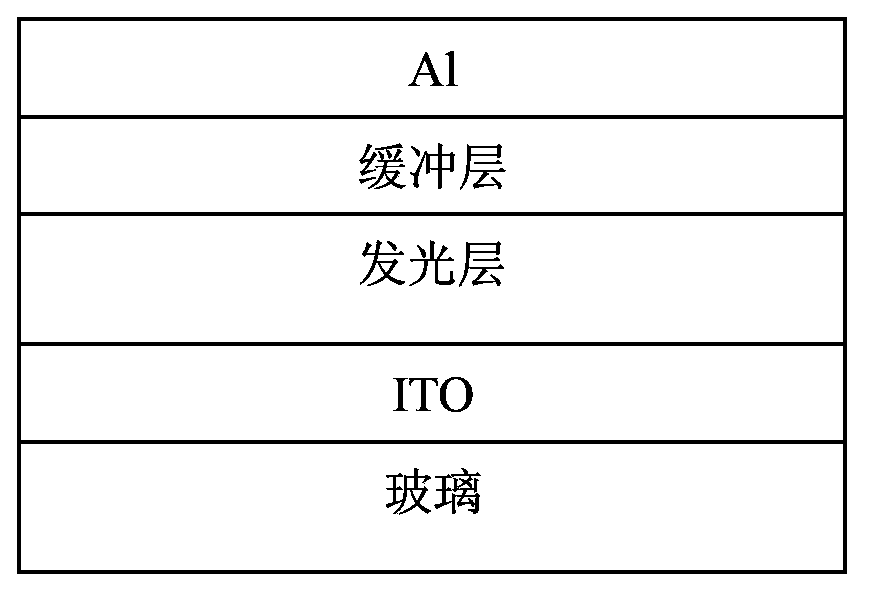
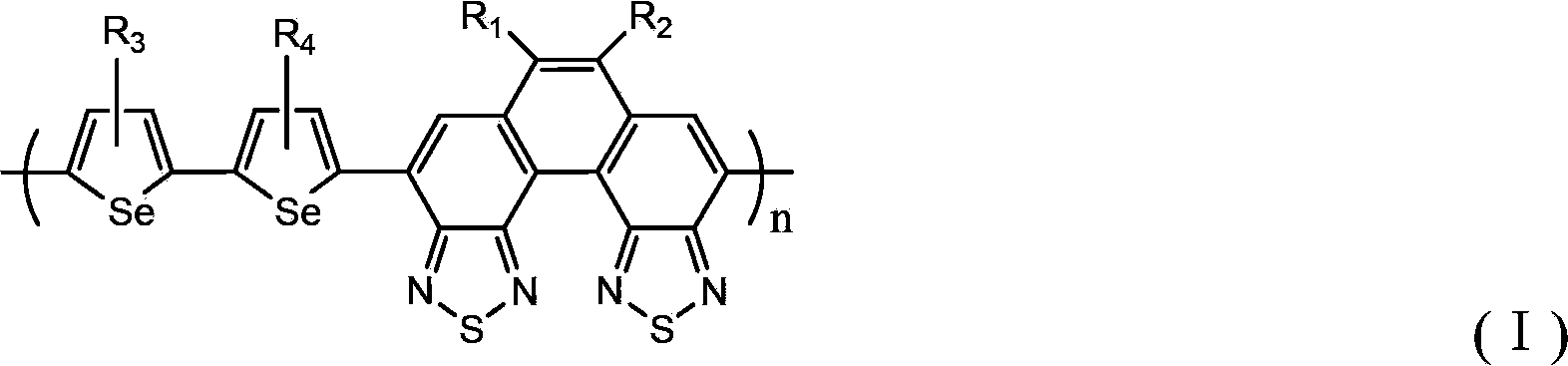
Biselenophen-benzobi(diazosulfide) copolymer and preparation method and application thereof
A benzothiadiazole and copolymer technology, which is used in semiconductor/solid-state device manufacturing, organic chemistry, electrical solid-state devices, etc., can solve problems such as limiting the scope of application, and achieve improved solubility and molecular weight. mature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method containing diselenophene-benzo two (benzothiadiazole) copolymers of the present invention comprises the following steps:
[0040] (1) In an inert gas environment, add 4,4'-dibromo-6,6'-diiodo-bis 2,1,3 benzothiadiazole, alkyne compound, first catalyst and base to the second In a solvent, react at 130° C. for 4 hours to obtain compound 3, the structural formula of which is
[0041] The molar ratio of the 4,4'-dibromo-6,6'-diiodo-linked 2,1,3 benzothiadiazole to the alkyne compound is 1:2, and the base is Bu 3 N, the molar ratio of the base to the alkyne compound is 1:1. The first catalyst is Pd(OAc) 2 (palladium acetate), the molar amount of the first catalyst is 4,4'-dibromo-6,6'-diiodo-linked 2,1,10% of 3-benzothiadiazole. The first solvent is DMF (N,N-dimethylformamide).
[0042] The reaction equation of this step is:
[0043]
[0044] (2) In an inert gas environment, add compound 4 to the second solvent, add butyllithium at -78°C for ...
Embodiment 1
[0055] The polymer of this example is: poly{2,2'-diselenophene-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e:3,4- e] bis(benzothiadiazole)}, having the structure of the formula:
[0056]
[0057] The preparation method of the polymer of the present embodiment comprises the following steps:
[0058] 1. Preparation of 4,4'-dibromo-6,6'-diiodo-linked 2,1,3 benzothiadiazole
[0059] (1) Preparation of 5-nitro-2,1,3 benzothiadiazole
[0060] In the three-necked flask, add 2-amino-5-nitroaniline (22.95g, 0.15mol) and 100ml thionyl chloride SOCl 2 , stirred and slowly added 2ml of pyridine dropwise, heated and refluxed at 80-90°C for 24 hours, stopped the reaction, heated to 80°C and rotary evaporated excess SOCl 2 Finally, the reaction product was cooled to room temperature, poured into a large amount of water, stirred, filtered, washed with water and dried in vacuum to obtain 21.7 g of the product 5-nitro-2,1,3-benzothiadiazole with a yield of 80%;
[0061]
[0062] (2) Preparat...
Embodiment 2
[0085] The polymer of this example is: poly{4,4'-didecyl-2,2'-diselenophene-6,7-bis(3,7-dimethyl)octyl-benzo[2 , 1-e:3,4-e]bis(benzothiadiazole)}, has the structure of the formula:
[0086]
[0087] The preparation method of the polymer of this embodiment comprises the following steps:
[0088] 1. Preparation of 4,9-dibromo-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e:3,4-e]bis(benzothiadiazole)
[0089] Same as Step 2 of Example 1.
[0090] 2. Preparation of 4,4'-didecyl-5,5'-bis(tributyltin base)-2,2'-diselenophene
[0091] Under the protection of nitrogen, add 4,4'-didecyl-5,5'-dibromo-2,2'-diselenophene (6.98g, 0.01mol) into the three-necked flask, add 80ml of tetrahydrofuran solvent , and slowly inject n-butyllithium (8.4mL, 2.5M, 0.02mol) with a syringe at -78°C, continue stirring for 1h, and inject tributyltin chloride (5.6 mL, 0.02mol), reacted for 1 hour, then warmed up to room temperature and stirred for 6 hours. Saturated aqueous sodium chloride solution (30ml) wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com