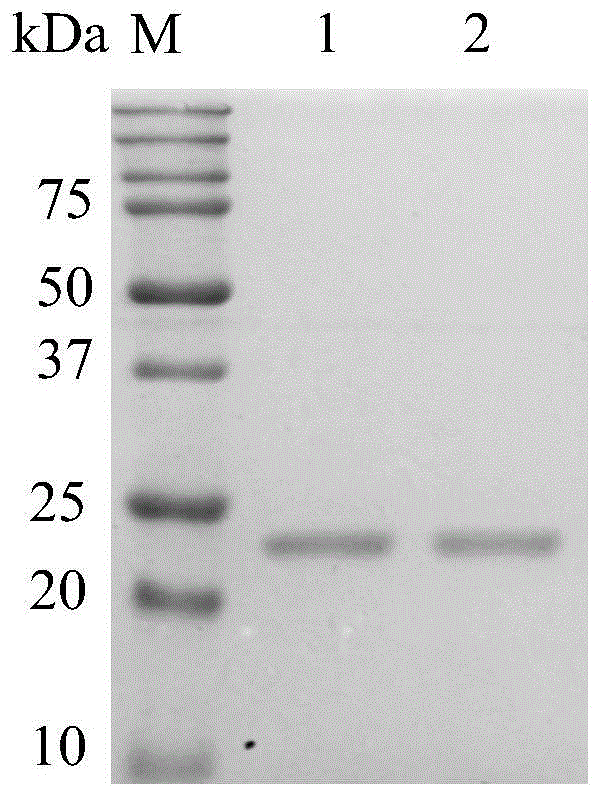
A kind of recombinant trypsin purification method
A trypsin and purification method technology, applied in biochemical equipment and methods, recombinant DNA technology, microorganism-based methods, etc., can solve the industrial application of histidine tags, the histidine tag cannot be completely removed, and is not suitable for large-scale applications. Large-scale production and other problems, to achieve the effect of saving purification steps, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 contains the preparation of the fermented liquid of recombinant trypsinogen
[0051] The nucleotide sequence encoding the porcine trypsinogen gene was synthesized by ligation PCR, and the PCR fragment was double digested with Xho I and EcoR I, inserted into the expression vector pPIC9K plasmid, and the recombinant plasmid pPIC9K-trypsin was constructed. In the recombinant plasmid, the expression of the target gene is carried out under the control of the AOX1 promoter, and there is an α signal peptide in front of the target gene, which can secrete the recombinantly expressed trypsinogen into the culture medium.
[0052] After the constructed recombinant plasmid was digested with Sac I, the recombinant plasmid was transformed into competent yeast cells by means of electroporation to construct a recombinant strain. The constructed recombinant strains were inoculated on the YPD solid medium containing G418, and positive strains were screened, and the screened p...
Embodiment 2
[0054] Activation of embodiment 2 recombinant trypsinogen
[0055] Take the fermented liquid provided by Example 1 of the present invention and carry out microfiltration and take the supernatant, add CaCl to the supernatant 2 to a final concentration of 10 mmol / L, adjust the pH of the fermentation broth to 7.0, and activate at room temperature with low-speed stirring for 24 hours to obtain a solution containing recombinant trypsin.
Embodiment 3
[0056] Activation of embodiment 3 recombinant trypsinogen
[0057] Take the fermented liquid provided by Example 1 of the present invention and carry out microfiltration and take the supernatant, add CaCl to the supernatant 2 to a final concentration of 50 mmol / L, adjust the pH of the fermentation broth to 8.0, and activate at room temperature with low-speed stirring for 8 hours to obtain a solution containing recombinant trypsin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com