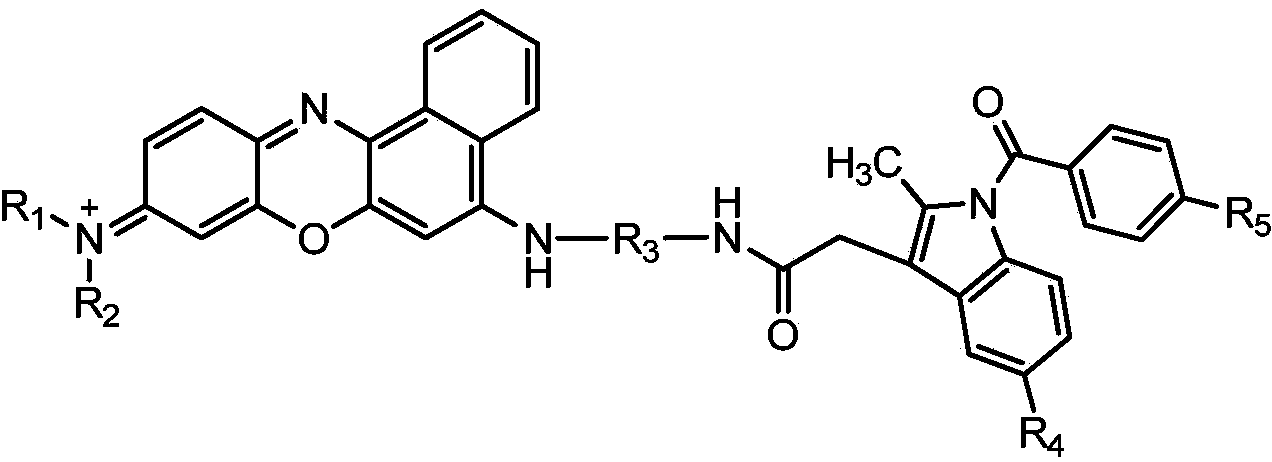
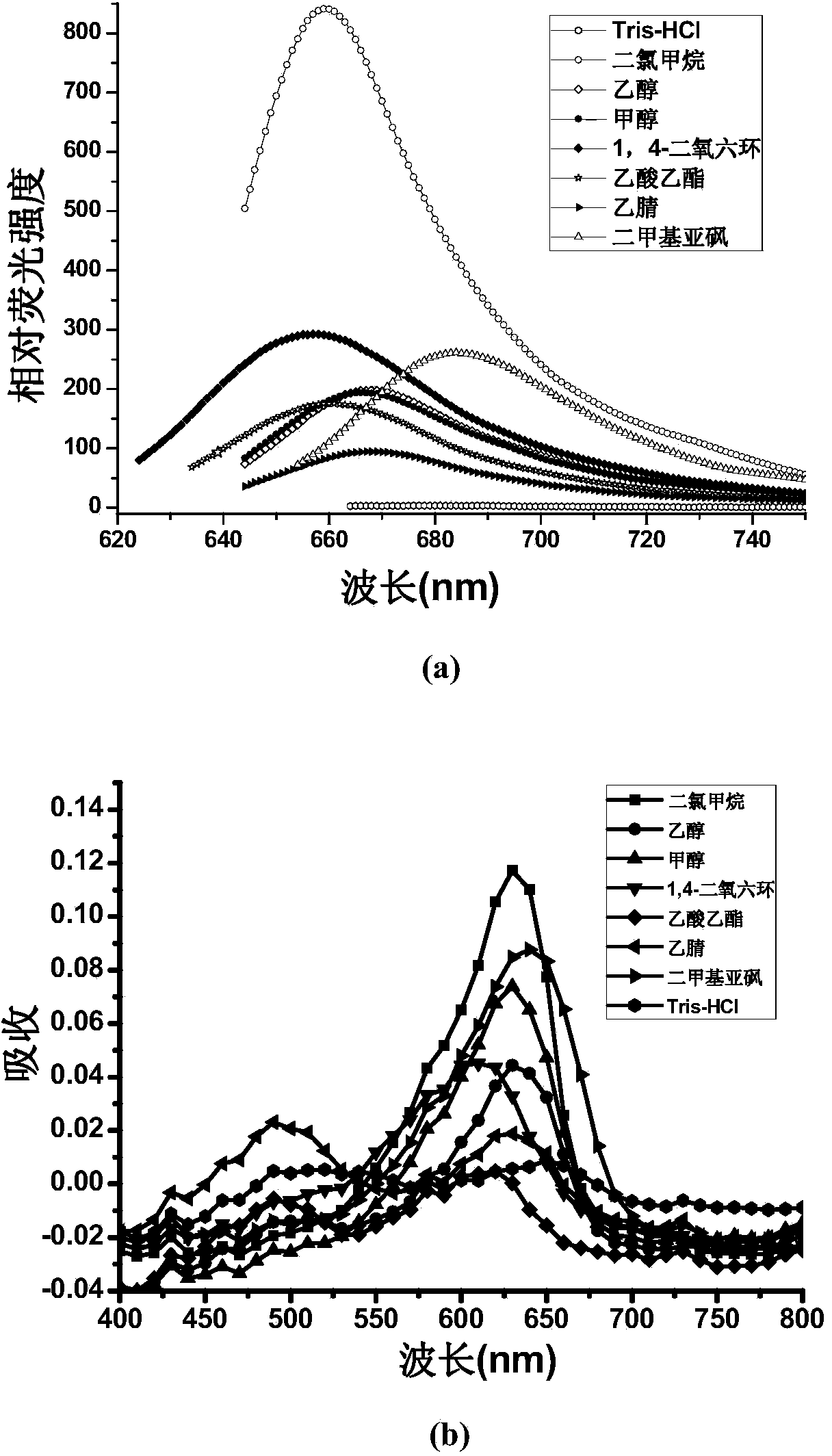
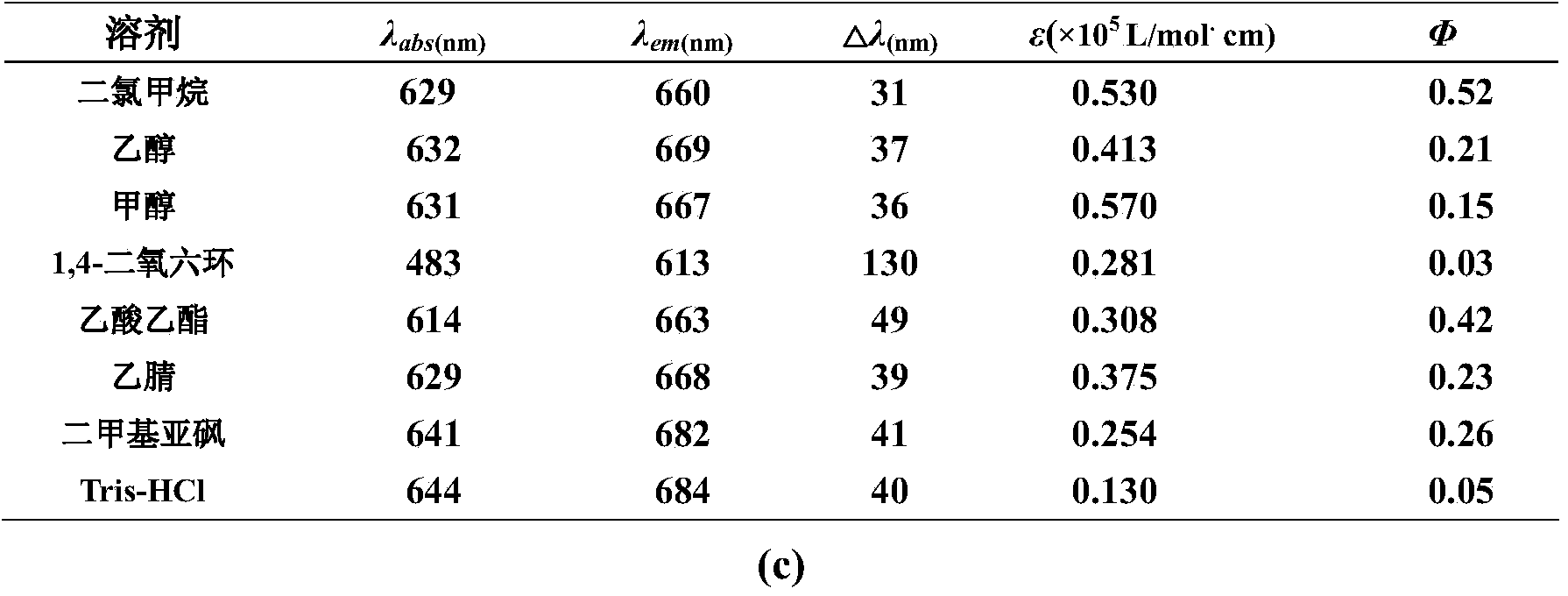
Near infrared fluorescence probe adopting nile blue as parent, preparation method thereof and applications thereof
A fluorescent probe, Nile blue technology, applied in the field of near-infrared fluorescent probes, can solve the problems of lack of imaging specificity, increase accurate diagnosis, large radioactivity, etc., achieve good cell membrane permeability, low biological toxicity, synthetic easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] On the other hand, the present invention provides the preparation method of the near-infrared fluorescent probe of the present invention using Nile Blue as a parent, comprising the steps of:
[0047] 1) 1-bromonaphthalene and H 2 N-R 3 -NH 2 According to the molar ratio of 1:1-1:5 reaction, the preparation of compound Ⅲ:
[0048]
[0049] The reaction temperature is 80-150°C, the reaction time is 1-24 hours, and the reaction solvent is selected from dichloromethane, ethylene glycol monomethyl ether, methanol, DMF or a mixture thereof;
[0050] In a preferred embodiment, the reaction temperature is 90-140°C, the reaction time is 10-20 hours, the reaction solvent is selected from ethylene glycol monomethyl ether, methanol, DMF or a mixture thereof, 1-bromonaphthalene and H 2 N-R 3 -NH 2 Mole is 1:1-1:4;
[0051] In a further preferred embodiment, the reaction temperature is 100-130°C, the reaction time is 12-18 hours, the reaction solvent is selected from ethylen...
Embodiment 1
[0077] 1.1 Synthesis of fluorescent probe compound II:
[0078]
[0079] (1) Synthesis of intermediate 1
[0080] N,N-Dimethyl-m-aminophenol (62.72mmol) was added to a round bottom flask containing a mixed solution of 30ml of concentrated hydrochloric acid and 10mL of water, and the temperature was kept at -5°C in an ice bath. Formulated with NaNO 2 (4.36g, 63.20mmol) in 30mL aqueous solution, and slowly added dropwise to the flask, mechanically stirred for 2h, filtered to obtain a brownish yellow solid, washed with 100mL saturated aqueous sodium acetate solution to obtain dark red solid intermediate 1, yield 86%.
[0081] (2) Synthesis of intermediate 2
[0082] 1-Bromonaphthalene (4.12 g) and hexamethylenediamine (4.65 g) were added to a round bottom flask containing 40 ml of ethylene glycol monomethyl ether solution, followed by CuI (190 mg) and CsCO 3 (3.0g), the solution changed from brown-yellow to blue-green. Heating to 125°C and reflux continued for 24 hours to ...
Embodiment 2
[0107] 2.1 Synthesis of fluorescent probe compound II':
[0108]
[0109] (1) Synthesis of Intermediate 1
[0110] N,N-Dimethyl-m-aminophenol (62.72mmol) was added to a round bottom flask containing a mixed solution of 30ml of concentrated hydrochloric acid and 10mL of water, and the temperature was kept at -5°C in an ice bath. With NaNO 2 (4.36g, 63.20mmol) in 30mL aqueous solution, and slowly added dropwise to the flask, mechanically stirred for 2h, filtered to obtain a brownish yellow solid, washed with 100mL saturated aqueous sodium acetate solution to obtain dark red solid intermediate 1, yield 86%.
[0111] (2) Synthesis of intermediate 2
[0112] 1-Bromonaphthalene (3.11 g) and hexamethylenediamine (2.64 g) were added to a round bottom flask containing 40 ml of ethylene glycol monomethyl ether solution, followed by CuI (190 mg) and CsCO 3 (3.0g), the solution changed from brown-yellow to blue-green. Heating to 125°C and reflux continued for 24 hours to stop the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com