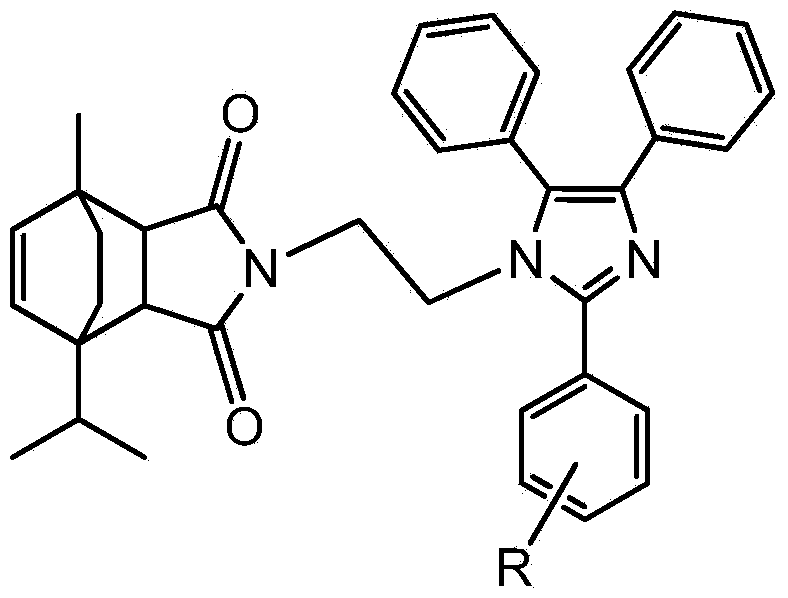
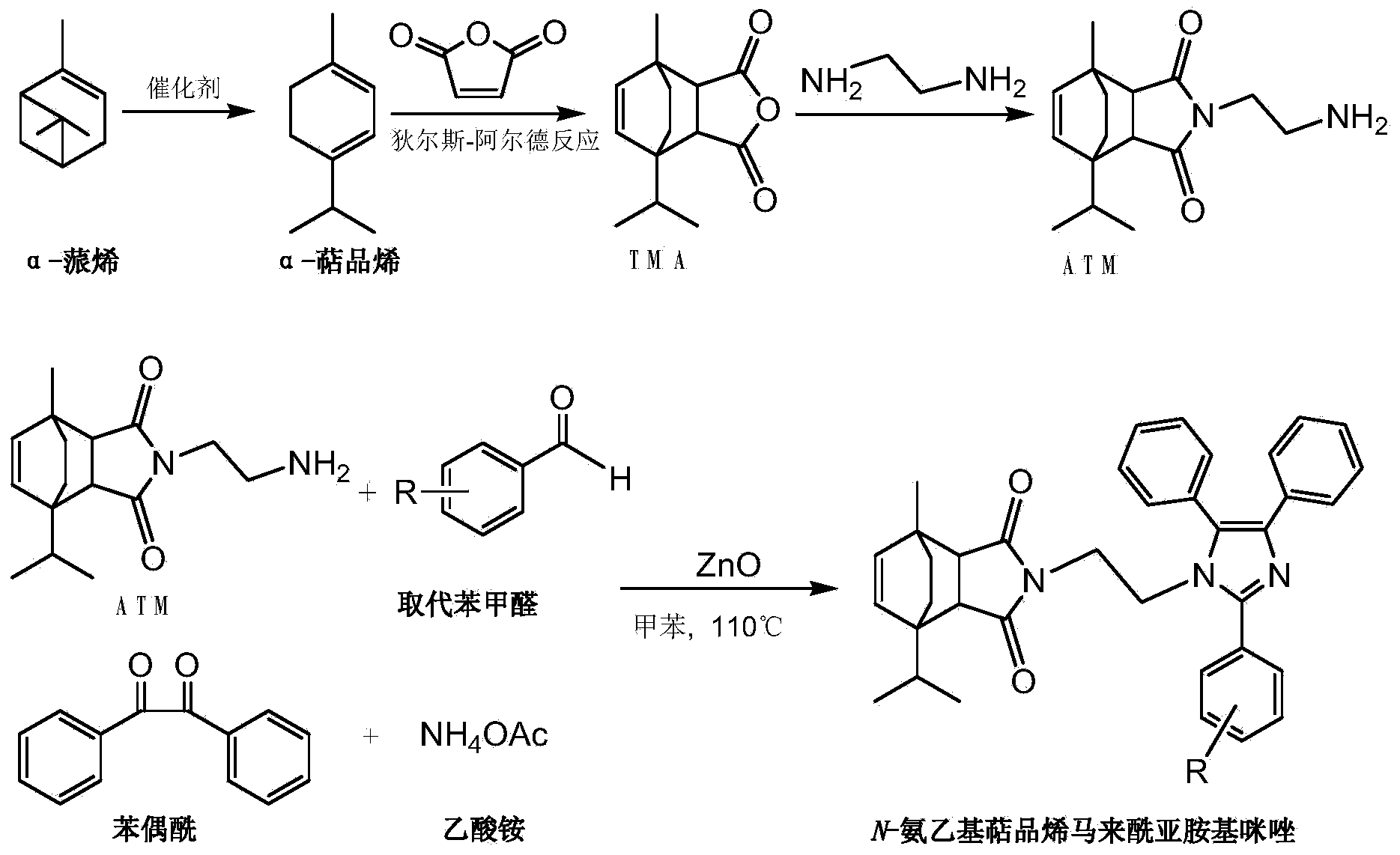
Synthesis method of N-aminoethyl terpinene maleimido imidazole derivative
A technology of enemaleimide imidazole and aminoethyl terpine, which is applied in the field of synthesis of N-aminoethyl terpinene maleimide imidazole derivatives, can solve problems that have not been reported at home and abroad. and other issues to achieve the effect of increasing added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of compound a:
[0024]
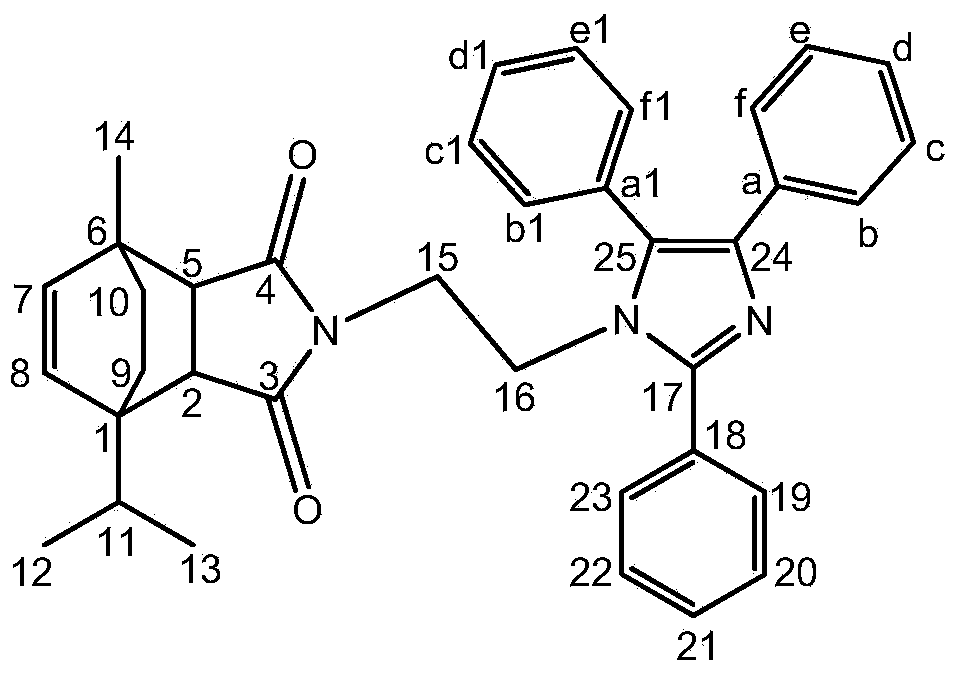
[0025] Add 0.6g ATM, 0.4g benzil, 0.26g benzaldehyde, 1.5g NH to the there-necked flask 4 OAc, 0.032g ZnO and 5ml toluene were stirred and refluxed for 2h, the reaction was terminated when no ATM was detected by TLC and ninhydrin hydrate, the solvent was evaporated, and the crude product was purified by silica gel column, and the eluent was petroleum ether / ethyl acetate: 10 / 1, 5 / 1, 3 / 1 gradient elution, and then recrystallized from a mixed solution of dichloromethane and petroleum ether to obtain the target product a. White crystal, m.p.187~188℃. IR(KBr,cm -1 )ν: 3030(Ar-H), 2958, 2869(C-H), 1771, 1700(C=O), 1601(Ar-C=C), 1502(C=N), 1125(C-N). 1 H NMR (600MHz, CDCl 3 )δ / ppm: 7.73~7.71(m, 2H, C 19 -H,C 23 -H),7.52~7.49(m,5H,C b1 -H,C c1 -H,C d1 -H,C e1 -H,C f1 -H),7.49~7.46(m,4H,C 20 -H,C 22 -H,C b -H,C f -H),7.45(t,J=7.4Hz,1H,C 21 -H),7.19(t,J=7.5Hz,2H,C c -H,C e -H),7.13(t,J=7.3Hz,1H,C d -H),5.74(d,J=8.4Hz,1H...
Embodiment 2
[0027] Preparation of compound b:
[0028]
[0029] Add 0.6g ATM, 0.4g benzil, 0.3g p-fluorobenzaldehyde, 1.5g NH to the there-necked flask 4OAc, 0.032g ZnO and 5ml toluene were stirred and refluxed for 2h, the reaction was terminated when no ATM was detected by TLC and ninhydrin hydrate, the solvent was evaporated, and the crude product was purified by silica gel column, and the eluent was petroleum ether / ethyl acetate: 10 / 1, 5 / 1, 3 / 1 gradient elution, and then recrystallized from a mixed solution of dichloromethane and petroleum ether to obtain the target product b. Light yellow powder, m.p.212~214℃. IR(KBr,cm -1 )ν: 3030(Ar-H), 2958, 2869(C-H), 1771, 1700(C=O), 1601(Ar-C=C), 1529(C=N), 1107(C-N). 1 H NMR (600MHz, CDCl 3 )δ / ppm: 7.73(m, 2H, C 19 -H,C 23 -H),7.50(m,5H,C b1 -H,C c1 -H,C d1 -H,C e1 -H,C f1 -H),7.47~7.44(m,2H,C b -H,C f -H),7.21(m,2H,C 20 -H,C 22 -H),7.19(m,2H,C c -H,C e -H),7.13(t,J=7.3Hz,1H,C d -H),5.76(d,J=8.4Hz,1H,C 8 -H),5.67(d,J=8.4H...
Embodiment 3
[0031] Preparation of compound c:
[0032]
[0033] Add 0.6g ATM, 0.4g benzil, 0.26g o-fluorobenzaldehyde, 1.5g NH to the there-necked flask 4 OAc, 0.032g ZnO and 5ml toluene were stirred and refluxed for 2h, the reaction was terminated when no ATM was detected by TLC and ninhydrin hydrate, the solvent was evaporated, and the crude product was purified by silica gel column, and the eluent was petroleum ether / ethyl acetate: 10 / 1, 7 / 1, 5 / 1, 3 / 1, 2 / 1 gradient elution, and then recrystallized with a mixed solution of dichloromethane and petroleum ether to obtain the target product c. Light yellow powder, m.p.65~68℃. IR(KBr,cm -1 )ν: 3039(Ar-H), 2958, 2869(C-H), 1771, 1700(C=O), 1601(Ar-C=C), 1580(C=N), 1017(C-N). 1 H NMR (600MHz, CDCl 3 )δ / ppm:7.71(td,J=7.5,1.7Hz,1H,C 23 -H),7.51(dd,J=7.3,6.0Hz,5H,C b1 -H,C c1 -H,C d1 -H,C e1 -H,C f1 -H),7.49~7.48(m,2H,C b -H,C f -H),7.47(m,1H,C 21 -H),7.31(td,J=7.5,0.9Hz,1H,C 20 -H),7.21(m,1H,C 22 -H),7.18(d,J=7.7Hz,2H,C c -H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com