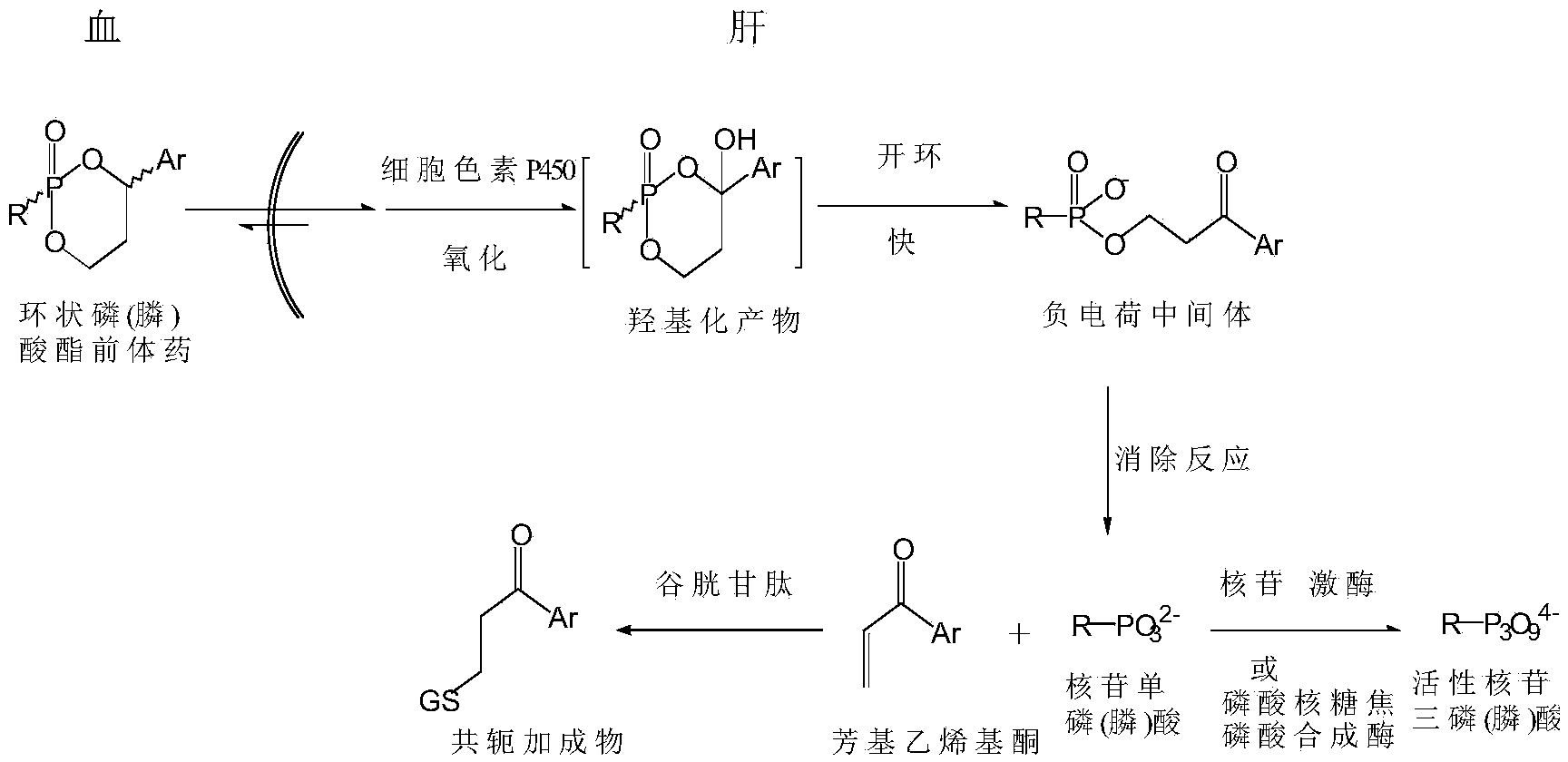
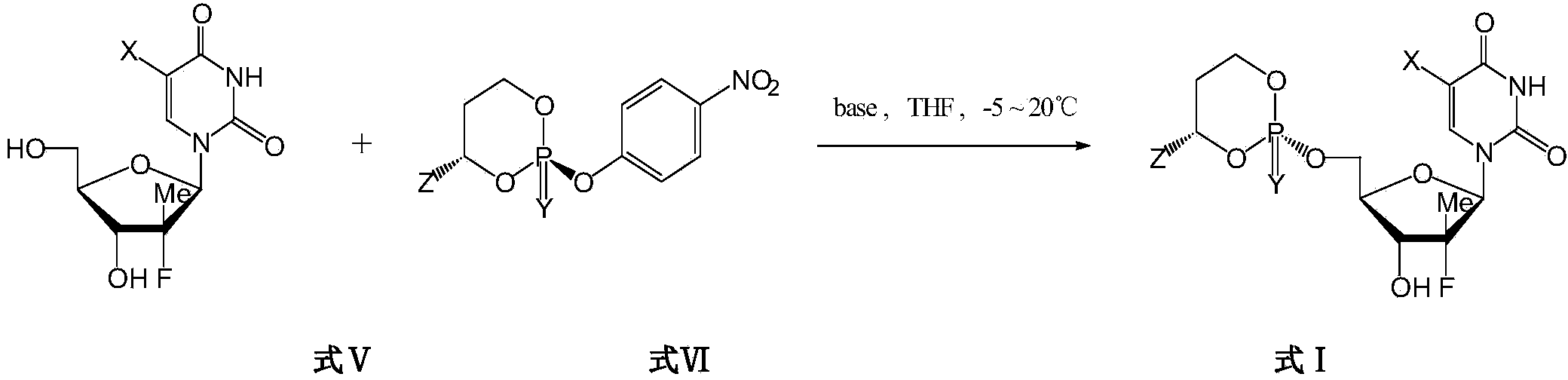
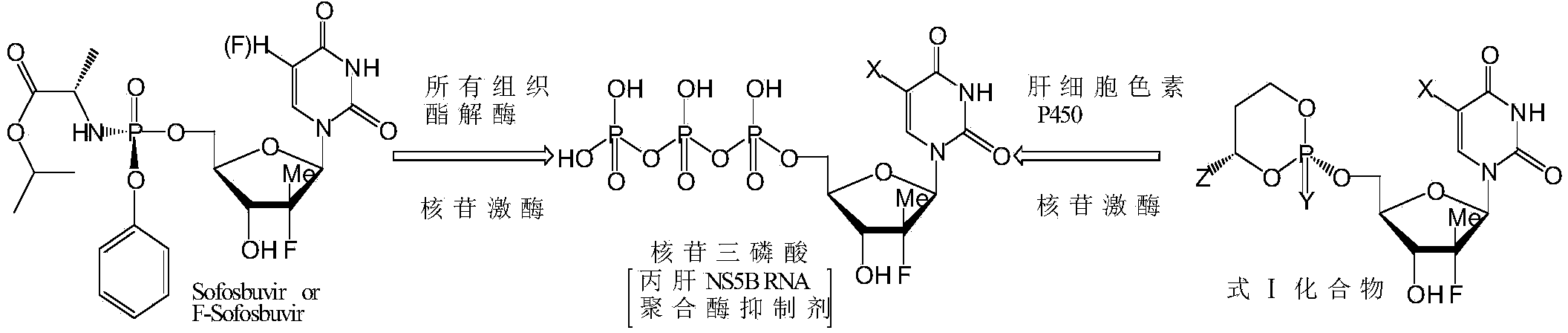
Cyclic nucleoside phosphate compound and preparation method and application thereof
A nucleoside cyclic phosphate ester and compound technology, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., to achieve good antiviral effects, small doses, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of Formula VIII Compounds
[0072]
[0073] The preparation method of the compound of the present embodiment formula VIII comprises the following steps:
[0074] 1) Suspend 20g of the compound of formula II in 300ml of anhydrous tetrahydrofuran under anhydrous, anaerobic, -5°C and stirring conditions to prepare a suspension of the compound of formula II, and then add dropwise 1M tert-butylmagnesium chloride ( t BuMgCl) tetrahydrofuran solution 160ml, at -5°C, after stirring for 0.5h, the temperature was raised to 20°C, continued stirring for 0.5h, the reaction mixture was cooled to 5°C, and a solution of 34g of the compound of formula VII dissolved in 200ml of tetrahydrofuran was added dropwise , at 5°C, after stirring for 20 h, the reaction mixture was cooled to -5°C, 80 ml of 2N hydrochloric acid aqueous solution was added dropwise, and the temperature was raised to room temperature under stirring to obtain a reaction solution;
[0075] 2) ...
Embodiment 2
[0080] Example 2 The preparation of formula IX compound
[0081]
[0082] The preparation method of formula IX compound, comprises the steps:
[0083] 1) Suspend 20g of the compound of formula III in 300ml of anhydrous tetrahydrofuran under anhydrous, anaerobic, -5°C and stirring conditions to prepare a suspension of the compound of formula II, and then add dropwise 1M tert-butylmagnesium chloride ( t BuMgCl) tetrahydrofuran solution 150ml, stirred at -5°C for 0.5h, then warmed up to 20°C, stirred for 0.5h, cooled the reaction mixture to 5°C, added dropwise a solution of 34g of the compound of formula VII dissolved in 200ml of tetrahydrofuran, stirred at 5°C for 20h Afterwards, the reaction mixture was cooled to -5°C, 80 ml of 2N hydrochloric acid aqueous solution was added dropwise, and the temperature was raised to room temperature under stirring to obtain a reaction solution;
[0084] 2) Remove the tetrahydrofuran in the reaction solution by rotary evaporation under r...
Embodiment 3
[0089] Example 3 Liver targeting evaluation of test compounds in mice
[0090] Referring to the method disclosed in Document 3 (Clin. Chem. 1992, 38, 480-485.), the liver targeting properties of the compounds to be tested (Sofosbuvir, Formula VIII, F-Sofosbuvir, Formula IX) in mice were comparatively studied.
[0091] After dissolving the compounds to be tested (Sofosbuvir, Formula VIII, F-Sofosbuvir and Formula IX) in dimethyl sulfoxide, they were diluted to the required concentration with phosphate buffer to prepare solutions of the compounds to be tested at the required concentrations.
[0092] Forty-eight BACLB / c mice weighing 20-25 g were randomly divided into four groups with 12 mice in each group. After 12 hours of fasting, 30 mg / Kg dose of the compound to be tested (that is, compound Sofosbuvir, formula VIII, F-Sofosbuvir and formula IX) solution was given by intragastric administration; after 60 minutes (min) and 120 minutes of administration, 6 The small intestine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com