Oxadiazole compound and preparation method thereof
A technology for oxadiazole compounds, which is applied in the field of oxadiazole compounds and their preparation, can solve problems such as application limitations, and achieve the effects of wide application range and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
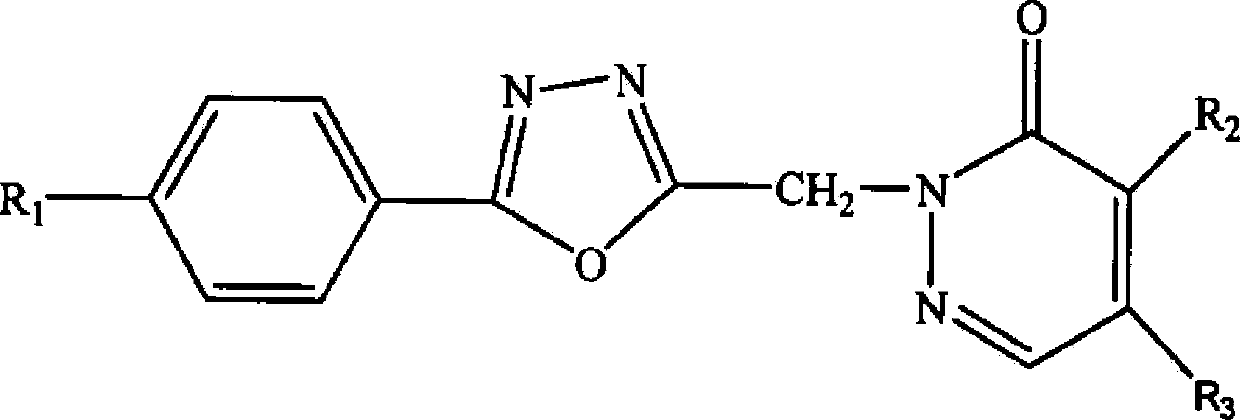
[0035] An oxadiazole compound, the compound molecular formula is as follows
[0036]
[0037] Among them, R 1 for hydrogen, R 2 is chlorine, R 3 For n-propylamine, the preparation method of this oxadiazole compound comprises the following steps:
[0038] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0039]
[0040] In a 250mL three-necked flask, add 50g of di-chlorobutenalonic acid and a small amount of water, stir to make an aqueous solution, then add 39g of hydrazine sulfate and 38.2g of sodium acetate, heat to 80-100°C, and react for 2 hours. After complete reaction, cool down, pour the reaction solution into cold water, a large amount of light yellow precipitate appears, filter it with suction, and dry it. The obtained product was recrystallized with absolute ethanol, and the yield was 89.5%.
[0041] (2) Synthesis of Benzohydrazide
[0042]
[0043] In a 250ml three-necked flask, add 70ml (0.485mol) of ethyl benzoate and 51.5ml (0.485mol) of hydrazine...
Embodiment 2
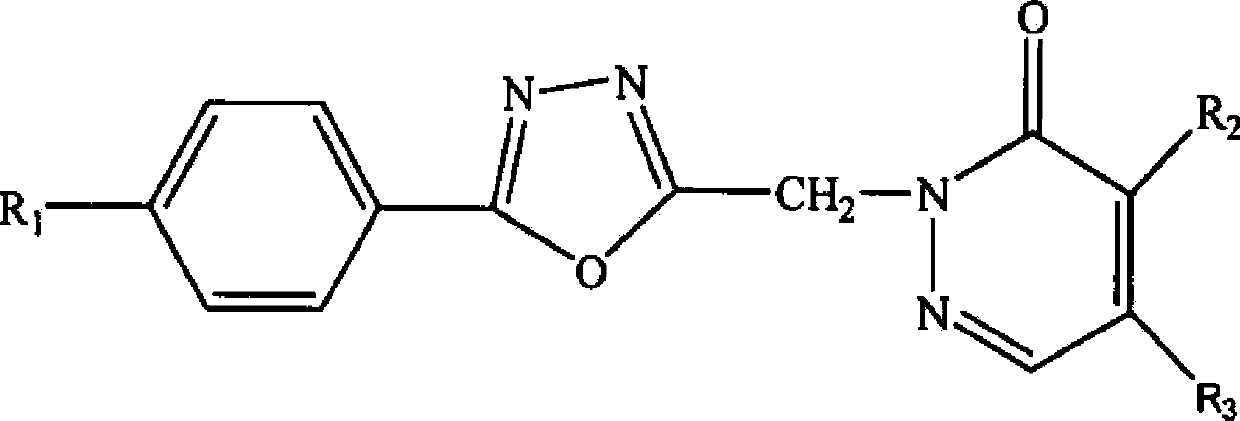
[0057] An oxadiazole compound, the compound molecular formula is as follows
[0058]
[0059] R 1 for hydrogen, R 2 is chlorine, R 3 Isopropylamine, the preparation method of this oxadiazole compound comprises the following steps:
[0060] (1) Preparation of 2-(substituted) aryl-5-chloromethyl-1,3,4-oxadiazole
[0061]
[0062] In a 250ml single-necked bottle equipped with a drying device, add 5g (0.024mol) of bishydrazide (N-p-methylbenzoyl-N'-chloroacetyl), 2.684ml (0.0288mol) of phosphorus oxychloride, Stir the reaction at 130° C. in an oil bath, and keep the temperature for 8 hours. After completion, the reaction solution was poured into 150ml of ice water, stirred and left for a period of time, a large amount of precipitation would occur, filtered to obtain a gray solid product, and the yield was 85.32%.
[0063] (2) Synthesis of 4,5-dichloro-2-(2-phenyl-5-methyl-1,3,4-oxadiazole)-3(2H)pyridazinone
[0064]
[0065] In a 250ml single-necked bottle, add 2-ph...
Embodiment 3
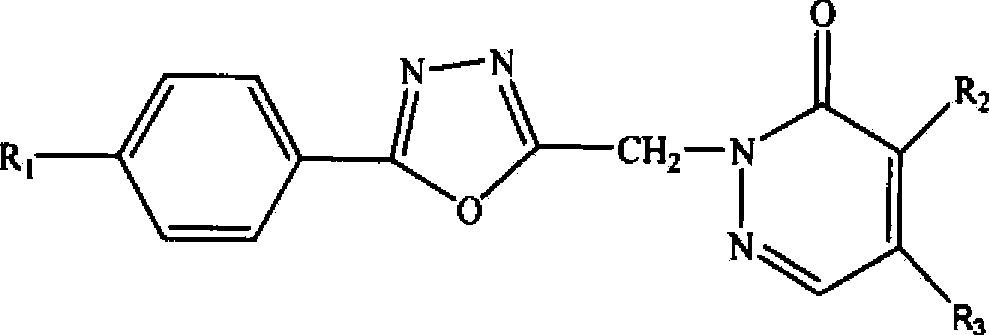
[0070] An oxadiazole compound, the compound molecular formula is as follows
[0071]
[0072] R 1 for hydrogen, R 2 is chlorine, R 3 For morpholine, the preparation method of this oxadiazole compound comprises the following steps:
[0073] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0074] Condensation of di-chlorocrotonic acid and hydrazine sulfate into 4,5-dichloro-3(2H)pyridazinone under the catalysis of sodium acetate;
[0075] (2) Preparation of Benzohydrazide
[0076] Ethyl benzoate and hydrazine hydrate were heated to reflux in ethanol solution and reacted for 8 hours to produce benzohydrazide, wherein the molar ratio of ethyl benzoate and hydrazine hydrate reaction was 1:2
[0077] (3) Preparation of bishydrazide
[0078] Mix the benzoyl hydrazide and α-haloacetyl halide prepared in step (2) with a molar ratio of 1:1 in THF, and react at 50°C for 5 hours under the catalysis of alkaline reagent potassium carbonate to synthesize bis hydrazide. The mola...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com