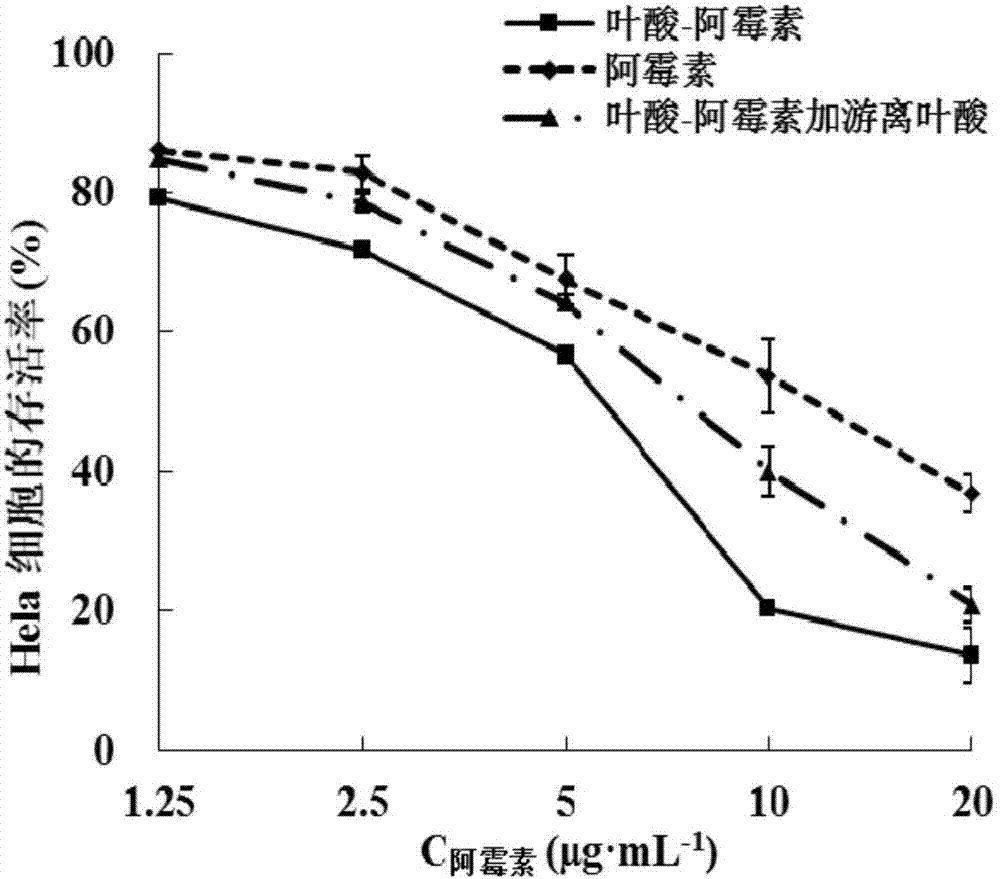
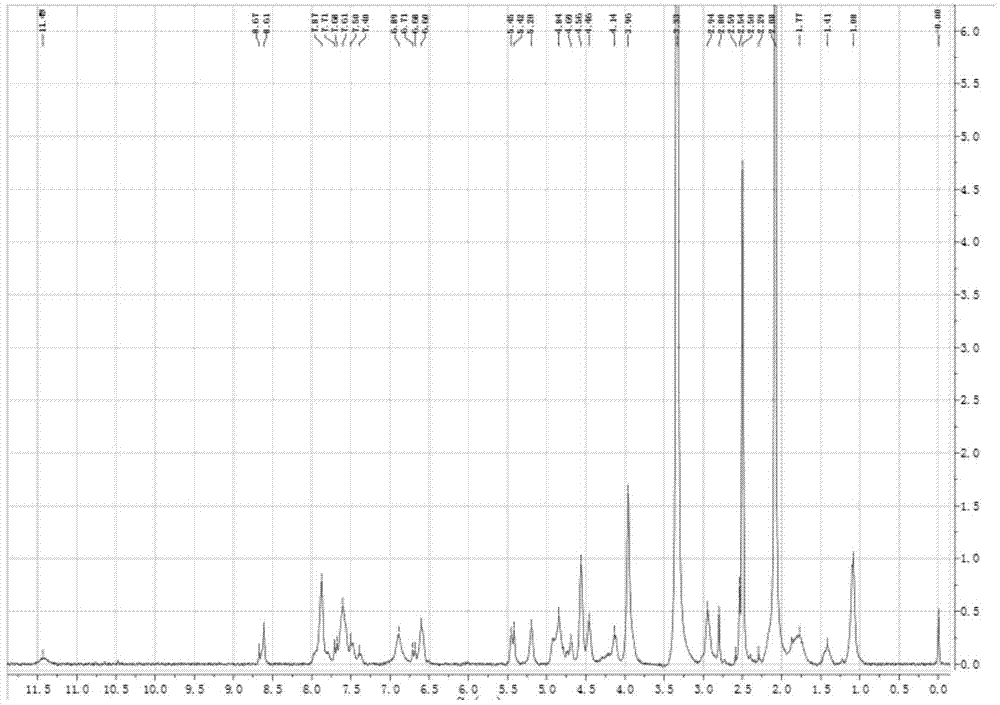
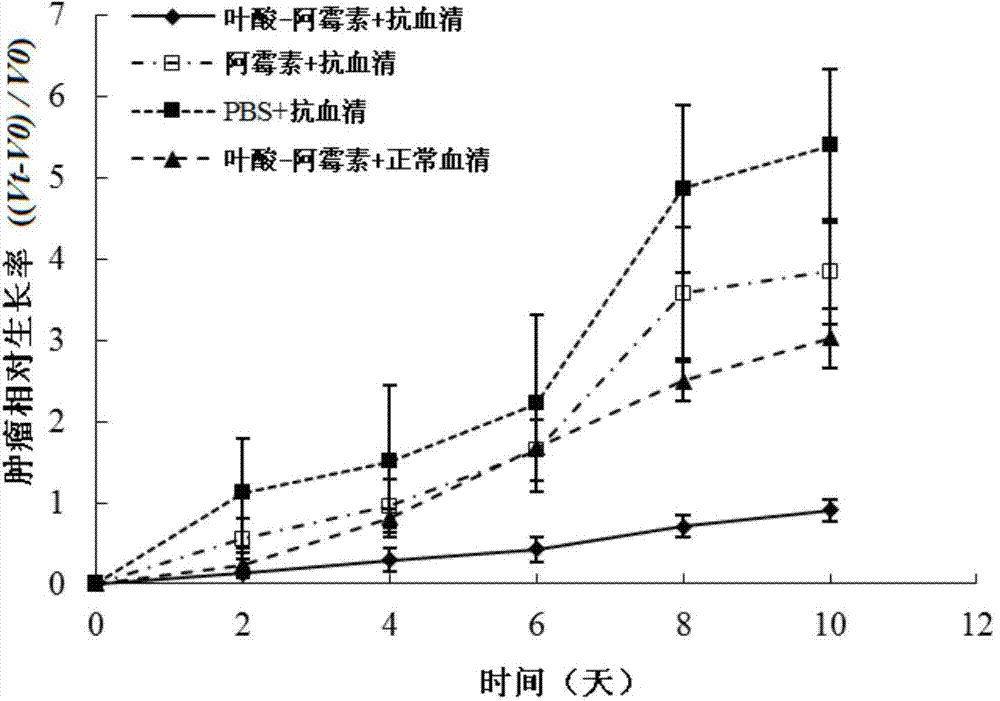
Folic acid-adriamycin immunological preparation for treating cancer through dual targets and preparation method thereof
A technology of doxorubicin and folic acid, which is applied in the preparation methods of peptides, chemical instruments and methods, and medical preparations of inactive ingredients, etc., can solve the problems of no cancer cells and long cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of doxorubicin-bovine serum albumin conjugate shown in formula I
[0056] Take 100mg of bovine serum albumin (BSA) in a 100mL round bottom flask, add 3mL of 0.1M phosphate buffered saline (PBS) to dissolve to obtain BSA solution; weigh another 78mg of doxorubicin (ADM) and add 1mL of DMF to dissolve, To obtain the ADM solution, add the ADM solution to the above-mentioned BSA solution, and slowly drop in 75 μL of 20-fold diluted 25% glutaraldehyde solution while stirring. After reacting in the dark for 5 hours at room temperature, the reaction solution was centrifuged at 6000 rpm and -4°C for 10 minutes. , transfer the supernatant to a dialysis bag (molecular weight cut-off 8000-12000), dialyze in 0.01M PBS dialysis medium for 7 days, and refresh the dialysis medium 3 times a day. Then, the liquid in the dialysis bag was centrifuged at 6000 rpm at -4°C for 10 min, and the supernatant was taken and freeze-dried to obtain an orange-red solid powder, w...
Embodiment 2
[0059] Example 2 Investigation of the Immunogenicity of Adriamycin-Bovine Serum Albumin Conjugates as Shown in Formula I
[0060] Healthy New Zealand rabbits (commonly used animals for the preparation of polyclonal antibodies) were used to prepare polyclonal antibodies, and the immunogenicity of ADM-BSA provided in Example 1 was investigated. A total of 5 immunizations were performed (the first immunization and 4 booster immunizations). The first immunization operation is: weigh 1-4 mg of ADM-BSA provided in Example 1 and dissolve it in 0.5-4 mL of normal saline, add 0.5-3 mL of Freund's complete adjuvant to make a mixture, and inject the mixed solution subcutaneously at multiple points on the back of the rabbit. Solution 0.5-2mL / rat; booster immunization procedure: the second immunization was performed 1-5 weeks after the first immunization, with Freund's incomplete adjuvant, and the rest were the same as the first immunization. The next immunization is carried out every 2-5 ...
Embodiment 3
[0064] Example 3 Investigation of the Immunogenicity of Adriamycin-Bovine Serum Albumin Conjugates as Shown in Formula I
[0065] Prepare polyclonal antibody with healthy Balb / c mice (the next step of dual targeting research experimental model animals), investigate the immunogenicity of ADM-BSA provided in Example 1 to healthy Balb / c mice, and immunize 3 times (first immunization and 2 booster immunizations). The first immunization operation is: weigh 1-2 mg of ADM-BSA provided in Example 1, dissolve it in 0.5-2 mL of normal saline, add 0.5-3 mL of Freund's complete adjuvant to make a mixture, and inject it subcutaneously at multiple points on the back of the mouse Mixed liquid 0.05-1mL / mouse; three booster immunizations (due to the longevity of mice and the need for later treatment, 2 immunizations less than rabbits, but it will affect the activity of the antibody obtained), the specific operation is: perform the second immunization 1 to 3 weeks after the first immunization. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com