Preparation method for delafossite-structure AgCrO2 nanocrystalline material
A nanocrystalline material, delafossite technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problem that the nanoeffect of photoelectrochemical properties has not been fully reflected and has not reached the nanometer scale , large crystal size and other problems, to achieve the effect of easy control of process parameters, low price and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
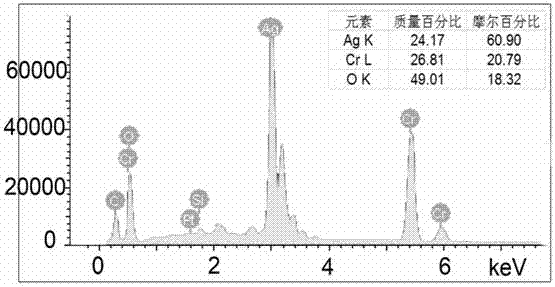
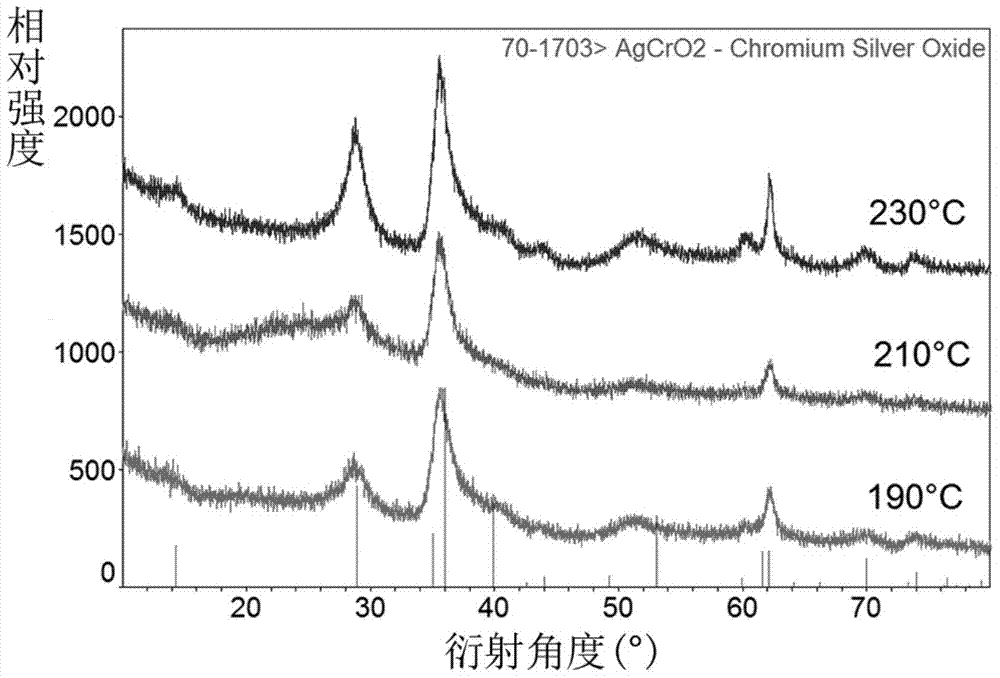
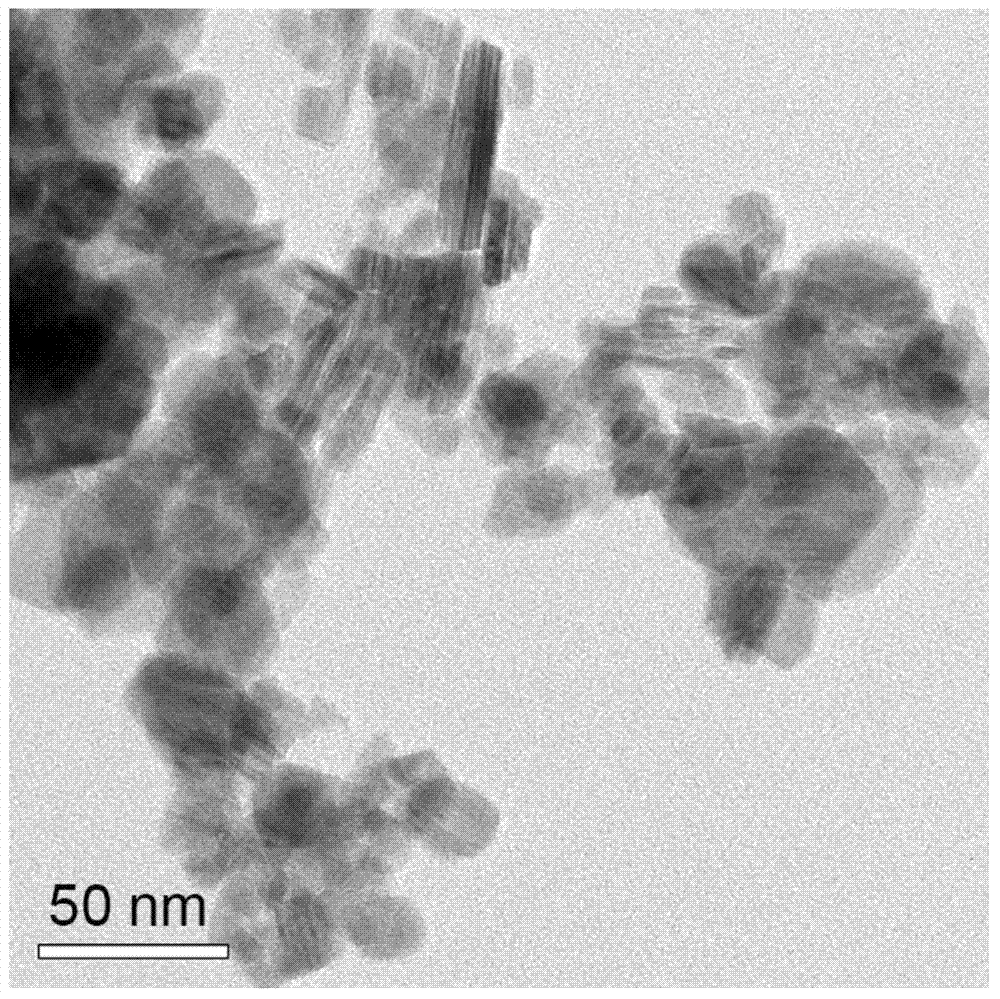
[0027] Weigh AgNO at room temperature according to the Ag:Cr molar ratio of 0.9 to 1.1:1 3 and Cr(NO 3 ) 3Finally, add deionized water and stir it with a magnetic stirrer for about 10-15 minutes. After it is completely dissolved, add 4 times the molar amount of NaOH that acts as a mineralizer, and continue stirring for about 10-15 minutes until it is completely dissolved and forms water. Thermally reactive precursor. Transfer the above reaction precursor to a hydrothermal reactor (usually polytetrafluoroethylene), and control the filling rate of the reaction solution to about 65%. After sealing the kettle body, place it in a programmed temperature-controlled oven for hydrothermal reaction, set the reaction temperature to 230°C, and the reaction time to 36-60 hours.
[0028] After the reaction, the kettle body was naturally cooled to room temperature, and the kettle body was opened to take out the reaction product. Use deionized water followed by dilute HNO 3 , dilute NH ...
Embodiment 2
[0030] Weigh the AgNO at room temperature according to the Ag:Cr molar ratio of 1:1 3 and Cr(NO 3 ) 3 Finally, add deionized water and stir it with a magnetic stirrer for about 10-15 minutes. After it is completely dissolved, add 4 times the molar amount of NaOH that acts as a mineralizer, and continue stirring for about 10-15 minutes until it is completely dissolved and forms water. Thermally reactive precursor.
[0031] Transfer the above reaction precursor to a hydrothermal reaction kettle (usually polytetrafluoroethylene), and control the filling rate of the reaction solution to about 65-75%. After sealing the kettle body, place it in a programmed temperature-controlled oven for hydrothermal reaction, set the reaction temperature to 210°C, and the reaction time to 36-60 hours.
[0032] After the reaction, the kettle body was naturally cooled to room temperature, and the kettle body was opened to take out the reaction product. Use deionized water followed by dilute HNO ...
Embodiment 3
[0034] Weigh the AgNO at room temperature according to the Ag:Cr molar ratio of 1:1 3 and Cr(NO 3 ) 3 Finally, add deionized water and stir it with a magnetic stirrer for about 10-15 minutes. After it is completely dissolved, add 3 times the molar amount of NaOH that acts as a mineralizer, and continue stirring for about 10-15 minutes until it is completely dissolved and forms water. Thermally reactive precursor.
[0035] Transfer the above reaction precursor to a hydrothermal reactor (usually polytetrafluoroethylene), and control the filling rate of the reaction solution to about 70%. After sealing the kettle body, place it in a programmed temperature-controlled oven for hydrothermal reaction, set the reaction temperature to 190°C, and the reaction time to 36-60 hours.
[0036] After the reaction, the kettle body was naturally cooled to room temperature, and the kettle body was opened to take out the reaction product. Use deionized water followed by dilute HNO 3 , dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com