Recombinant engineering bacteria for efficiently expressing human growth hormone, construction method and application
A technology of secretion expression and Escherichia coli, which is applied in the field of medical bioengineering, can solve the problems of low activity and low purity of hGH, and achieve the effect of simple purification process, simplified purification process and high expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: Construction of rhGH expression engineering bacteria
[0097] 1. Optimize the acquisition of genes
[0098] According to the known natural amino acid sequence of hGH, according to the codon preference of Escherichia coli and considering the elimination of secondary structures such as hairpin structures that are not conducive to expression, the coding sequence primers of hGH were designed without changing the amino acid sequence. The full-length sequence of rhGH was obtained by PCR method. The coding sequence of the Escherichia coli heat-stable enterotoxin signal peptide was synthesized by Bao Bio.
[0099] 2. Construction of expression plasmid pET22b(+)-rhGH and transformation of host bacteria
[0100] The expression plasmid is the pET22b(+) expression vector purchased from InVitrogen (the expression vector contains an IPTG-induced promoter and peIB secretion signal peptide), and the host bacteria is Escherichia coli BL21(DE3).
[0101] Treat the synthe...
Embodiment 2
[0104] The selection of embodiment 2 suitable expression vectors
[0105] According to the method similar to Example 1, insert the hGH sequence into several different expression vectors, such as pET28a(+) (purchased from Invitorgen Company), pET29a(+) (purchased from Invitorgen Company), pET-30Ek / LIC purchased from Invitorgen Company) the same restriction site (NcoI / HindIII), to obtain plasmid pET28a (+)-rhGH, pET29a (+)-rhGH, pET-30Ek / LIC-rhhGH.
[0106] The plasmids pET28a(+)-rhGH, pET29a(+)-rhGH, and pET-30Ek / LIC-rhGH were transformed into corresponding host bacteria respectively, and resistant strains were selected. Shake flask experiments were carried out together with engineering bacteria containing plasmid pET22b(+) / rhGH. After being induced by IPTG (or arabinose) for 2 hours, the expression of the target protein expressed by the engineering bacteria containing plasmid pET22b(+)-rhGH accounted for the total protein About 20%, while the expression of the target protein ...
Embodiment 3
[0107] The selection of embodiment 3 optimum culture medium
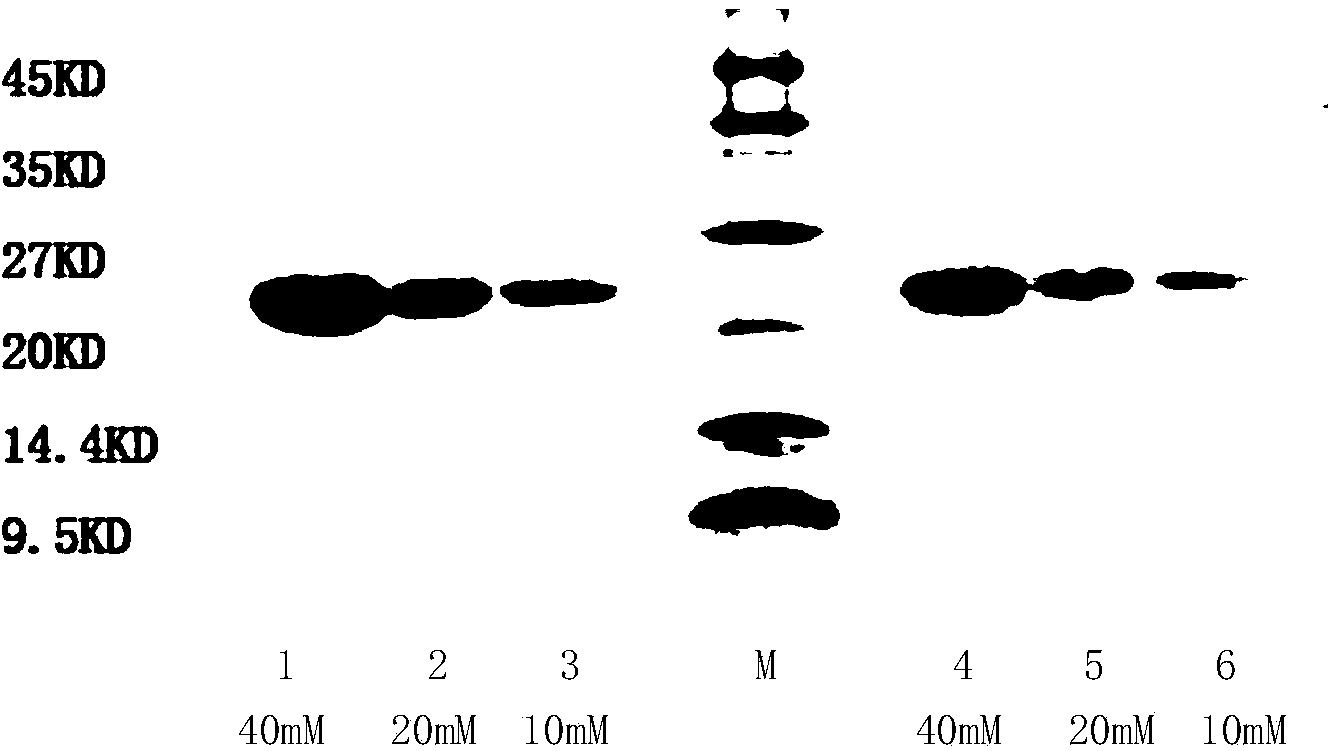
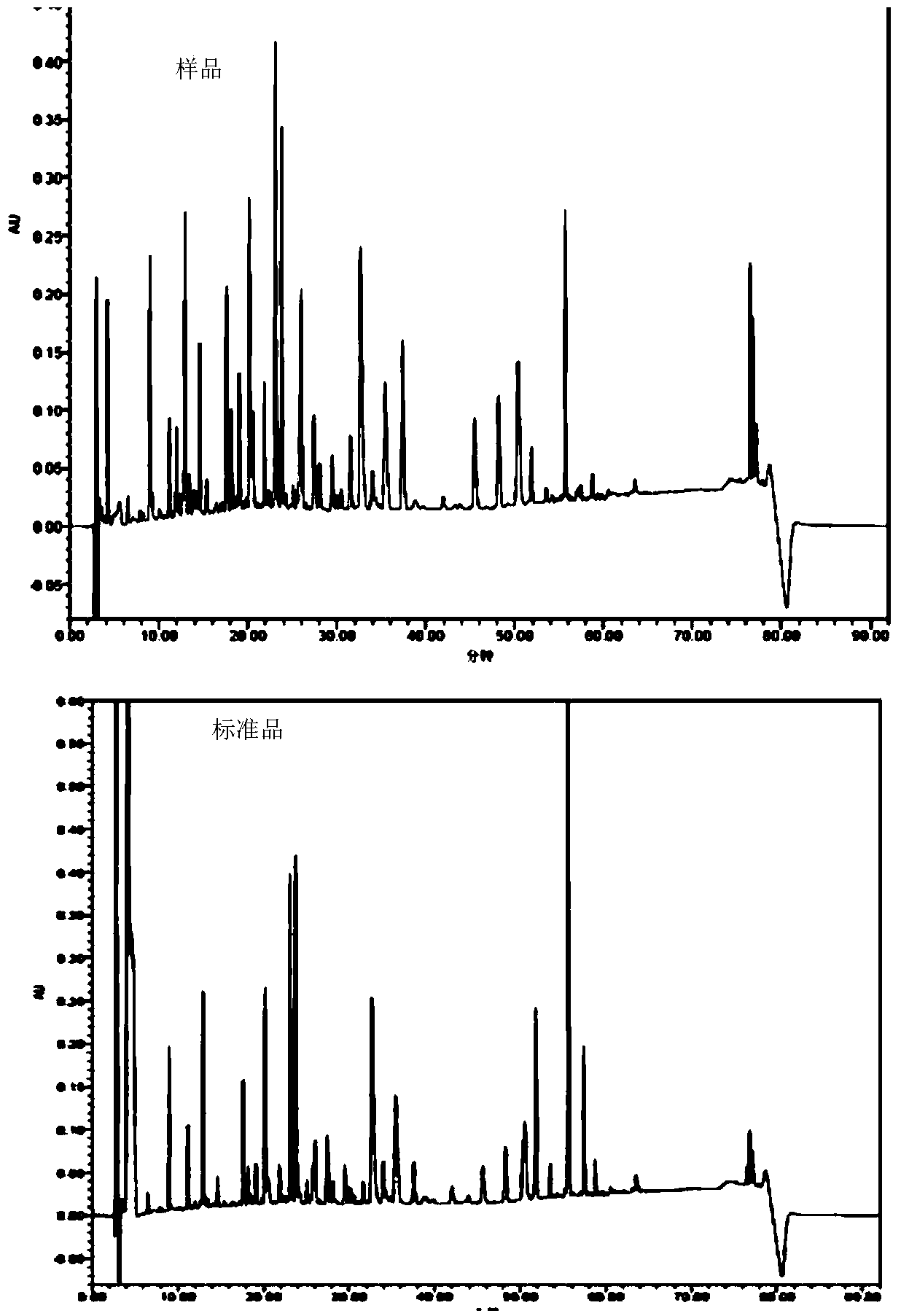
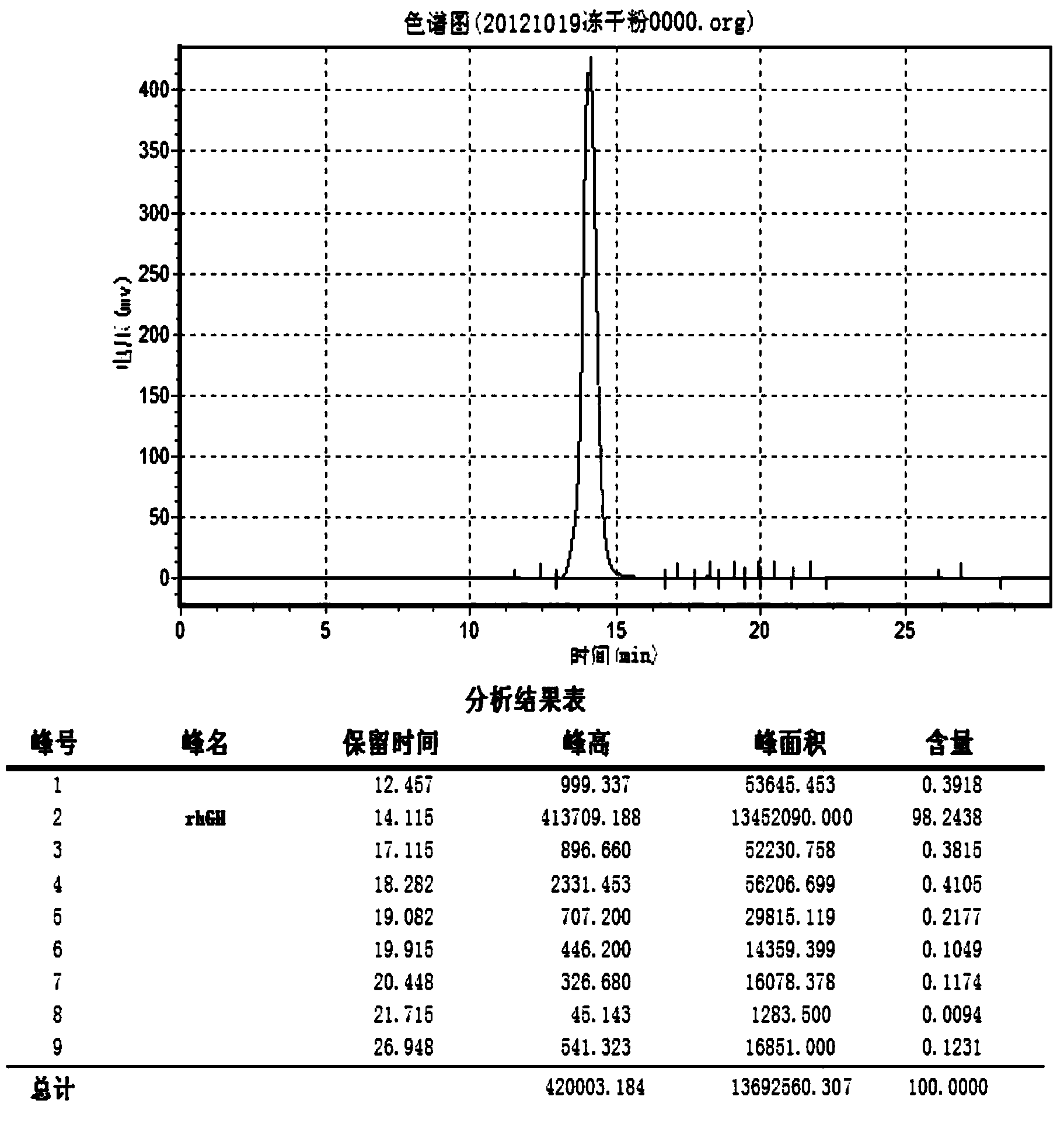
[0108] Pick the Escherichia coli BL21 (DE3) monoclonal that transfers to pET22b (+)-rhGH prepared in Example 1, inoculate in the 250ml Erlenmeyer flask that contains 50ml LB seed liquid, cultivate 3-4hr, wait for OD 600 reach 0.8-1.0, inoculate in a 1000ml Erlenmeyer flask containing 250ml improved seed solution at a ratio of 1:10, cultivate for about 8-10h, and wait until OD 600 When it reaches 6-8, ferment in a tank according to 1:10, add carbon source (glycerol), magnesium salt, ammonia water to adjust pH6.8-7.0, temperature 37°C, DO>30%, wait for OD 600 When it reaches 15-18, add 0.5-0.8mM IPTG to start induction, add carbon source (glycerol), nitrogen source, and phosphate buffer to adjust pH to 7.2-7.4, DO>50%, temperature 30-32°C, induction 4 -5hr over. The samples were tested by SDS-PAGE to determine the protein content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com