Novel cell-penetrating peptide
A membrane-penetrating peptide and cell technology, which is applied to peptides, bacteria, cells modified by introducing foreign genetic material, etc., can solve the problem of low membrane penetration efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: HBD mutant expression plasmid construction
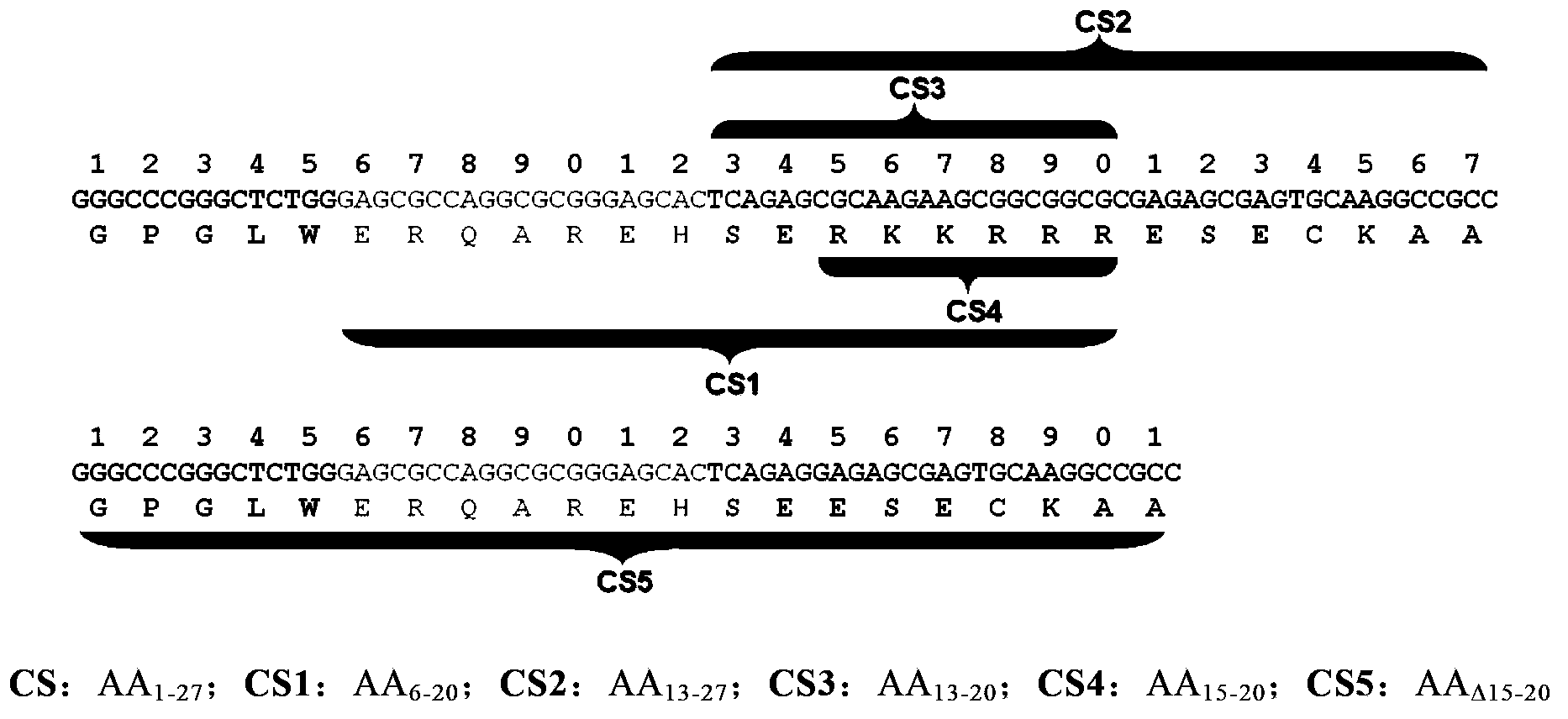
[0069] We constructed expression plasmids fused with EGFP and various mutated HBD sequences: EGFP-CS1-pET28a, EGFP-CS2-pET28a, EGFP-CS3-pET28a, EGFP-CS4-pET28a, EGFP-CS5-pET28a .
[0070] 1. Construction of EGFP-CS1 recombinant protein expression plasmid
[0071] Using the EGFP-pET28a plasmid as a template, primers were designed, and the sequence of CS1 was amplified by PCR to obtain the target band of EGFP with CS1, which was cloned into the pMD18T vector for amplification. The amplified plasmid DNA was extracted, and the target fragment was recovered by double digestion with NdeI and BamHI, and ligated with pET28a recovered by double digestion with the same two enzymes to construct a plasmid into an EGFP-CS1-pET28a expression vector ( figure 2 ).
[0072] PCR amplification primers for CS1
[0073] Forward: 5'-AAA CATATG GTGAGCAAGGGCGAGGAGC-3'
[0074] Reverse: 5'-AAA GGATCC TTAGCGCCGCCGCTTCTTGCGCTCTG...
Embodiment 2
[0108] Embodiment 2: Expression and purification of EGFP-HBD recombinant protein
[0109] Expression and purification of EGFP-CS1 (amino acid sequence described in SEQ ID NO.9), EGFP-CS2 (amino acid sequence described in SEQ ID NO.10), EGFP-CS3 (amino acid sequence described in SEQ ID NO.11) , EGFP-CS4 (amino acid sequence described in SEQ ID NO.8), EGFP-CS5 (amino acid sequence described in SEQ ID NO.12), EGFP-CS recombinant protein (amino acid sequence described in SEQ ID NO.13) , EGFP-TAT recombinant protein (amino acid sequence as described in SEQ ID NO.14).
[0110] Taking EGFP-CS4 (amino acid sequence as described in SEQ ID NO.8) as an example, transfer the plasmid EGFP-CS4-pET28a into BL21 (DE3), and culture it in 200ml medium at 37°C until OD 600 0.4-0.6, add IPTG (final concentration: 0.5mM), induce expression at 15°C for 12h, and ultrasonically break the cells. The ultrasound conditions are: 40w ultrasound for 4s, rest for 5s, ultrasound 70 times. The supernatant ...
Embodiment 3
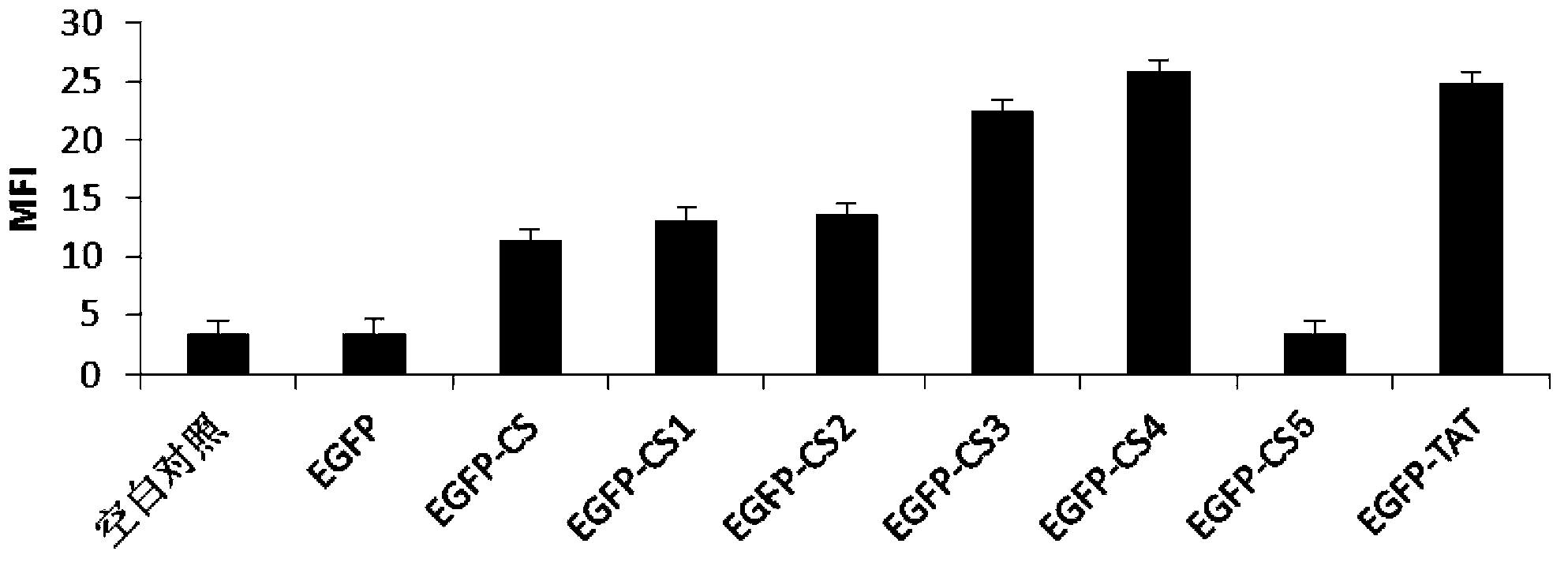
[0113] Example 3: Research on the Effect of Penetrating the Membrane
[0114] 3.1 Membrane penetration of EGFP-HBD recombinant protein
[0115] A549 lung cancer cells or HeLa cells in good growth state were treated with 1×10 5 / ml inoculated into the cell culture plate (put a sterile cover glass in each well), 2ml per well. Then place the cell plate at 37°C, 5% CO 2 Culture in an incubator with saturated humidity to make the cells adhere to the wall well.
[0116] When the cell coverage rate reaches about 50%, discard the medium in the culture plate, rinse the adherent cells with PBS three times, then add 2ml of new medium, and add the corresponding EGFP-CS mutant fusion protein to each well to The same amount of EGFP recombinant protein was added as a negative control, and the sample well without drug was used as a blank control, and placed in an incubator at 37°C and 5% CO2 saturated humidity for 13 hours. The medium was then aspirated, rinsed three times with PBS, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com