A kind of synthetic method of polysubstituted tetrahydroisoquinoline derivatives
A technology of tetrahydroisoquinoline and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of insufficient product structure diversity, narrow substrate applicability, harsh reaction conditions, etc., and achieves high atom economy, low cost, raw material easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
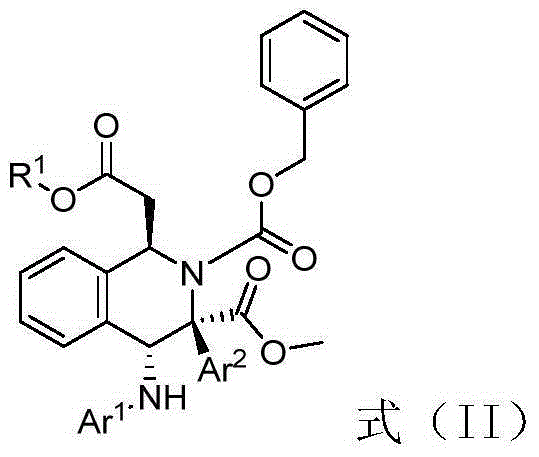
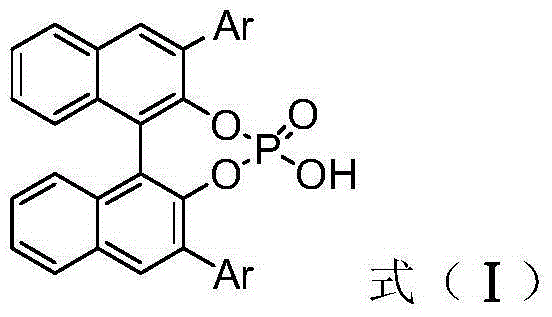
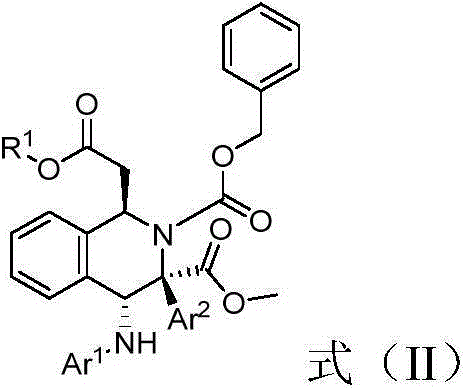
[0036] Weigh (E)-methyl-3-(2-formylphenyl) acrylate (0.22mmol), p-cymene dichloride ruthenium (0.004mmol), p-bromoaniline (0.2mmol), Molecular sieves (100mg), (S)-3,3'-phenyl substituted BINOL phosphoric acid (0.01mmmol), L-mandelic acid (0.02mmol) put them into a small test tube reactor, add redistilled 5.6 milliliters of 1,2-dichloroethane, stirred for one hour, then dropped to minus ten degrees Celsius, added benzyl carbamate (25.9 mg, 0.24 mmol), weighed methyl phenyldiazoacetate (0.24 mmol) and dissolved in 2.8ml of redistilled 1,2-dichloroethane, and injected into the reaction system through a peristaltic pump for 1 hour, after the injection was completed, potassium tert-butoxide (0.3mmol) was added and stirred for 1 hour, then filtered, and the filtrate was placed at 40°C The solvent is removed by rotary evaporation, and then separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1: 10 ~ 1: 5) to obtain a multi-substituted tetrahydroiso...
Embodiment 2
[0039]
[0040] Weigh (E)-methyl-3-(2-formylphenyl) acrylate (0.22mmol), p-cymene dichloride ruthenium (0.004mmol), p-bromoaniline (0.2mmol), Molecular sieves (100mg), (S)-3,3'-naphthyl substituted BINOL phosphoric acid (0.01mmmol), L-mandelic acid (0.02mmol) put them into a small test tube reactor, add redistilled 5.6 milliliters of 1,2-dichloroethane, stirred for one hour, then dropped to minus ten degrees Celsius, added benzyl carbamate (25.9 mg, 0.24 mmol), weighed methyl phenyldiazoacetate (0.24 mmol) and dissolved in 2.8ml of redistilled 1,2-dichloroethane, and injected into the reaction system through a peristaltic pump for 1 hour, after the injection was completed, potassium tert-butoxide (0.3mmol) was added and stirred for 1 hour, then filtered, and the filtrate was placed at 40°C The solvent is removed by rotary evaporation, and then separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1: 10 ~ 1: 5) to obtain a multi-substituted tetrahydro...
Embodiment 3
[0043]
[0044] Weigh (E)-methyl-3-(2-formylphenyl) acrylate (0.22mmol), p-cymene dichloride ruthenium (0.004mmol), p-bromoaniline (0.2mmol), Molecular sieves (100mg), (S)-3,3'-biphenyl substituted BINOL phosphoric acid (0.01mmmol), L-mandelic acid (0.02mmol) put them into a small test tube reactor, add redistilled 5.6 milliliters of 1,2-dichloroethane, stirred for one hour, then dropped to minus ten degrees Celsius, added benzyl carbamate (25.9 mg, 0.24 mmol), weighed methyl phenyldiazoacetate (0.24 mmol) In 2.8ml redistilled 1,2-dichloroethane, and injected into the reaction system by a peristaltic pump for 1 hour, after the injection was completed, potassium tert-butoxide (0.3mmol) was added and stirred for 1 hour, then filtered, and the filtrate was ℃ rotary evaporation to remove the solvent, and then separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1: 10 ~ 1: 5) to obtain multi-substituted tetrahydroisocyanate with high diastereoselectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com