Anthracycline antibiotic coupled nonlinear multi-block copolymer, and preparation method and application thereof
A technology of multi-block copolymers and antibiotics, applied in the direction of medical preparations, drug combinations, and pharmaceutical formulations of non-active ingredients, which can solve problems such as low solubility, limited application, and short half-life, and achieve easy dissolution and good therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
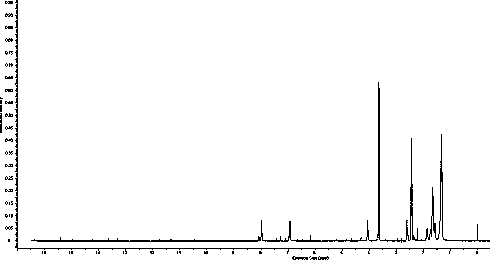
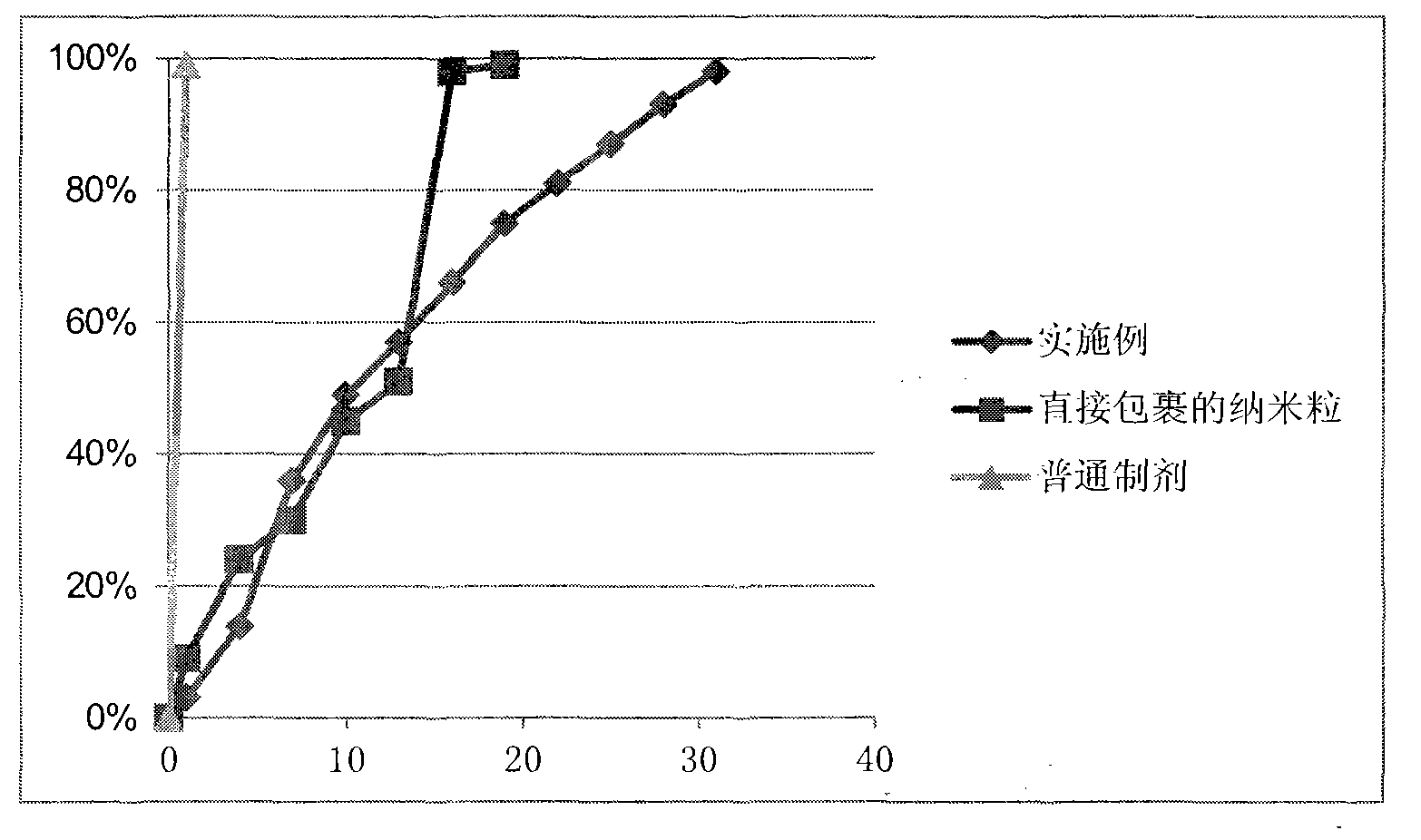
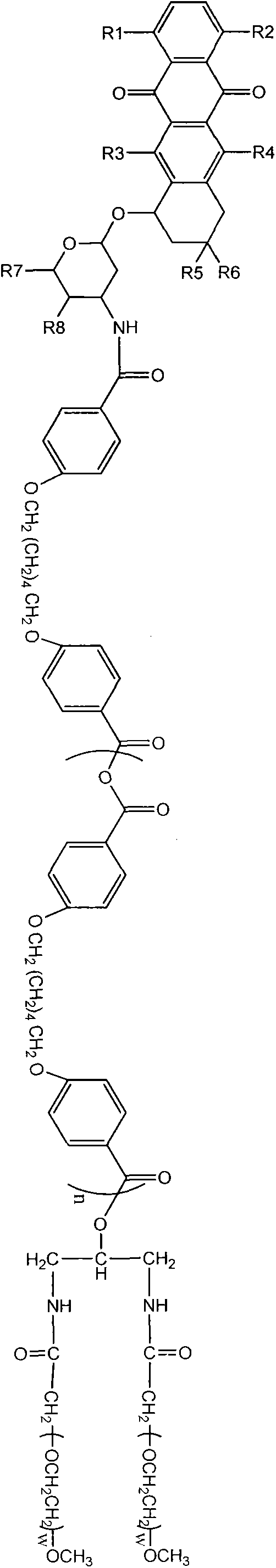
Image
Examples
Embodiment 1
[0054] 1) A mixture of 25 g of 1,6-bis(p-carboxyphenoxy)hexane in 500 ml of acetic anhydride is refluxed to form acetylated 1,6-bis(p-carboxyphenoxy)hexane;
[0055] 2) Mix 3 g of compound A, 51 mg of compound B, 165 mg of dicyclohexylcarbodiimide and 8 mg of pyridine, stir overnight at room temperature; then wash with ether and dry under vacuum to obtain a polymer;
[0056] 3) Mix the products of step 1) and step 2) into a flask, melt and polymerize under reduced pressure at 180°C for 1 hour; dissolve the polymer in chloroform after cooling to room temperature, wash with petroleum ether and dry;
[0057] 4) Put doxorubicin and the polymer of step 3 into 20ml dimethyl sulfoxide solution for 48 hours; then put it in an oven for 3 hours; at minus 10-20 degrees, ultrasonic for 15 minutes; homogenizer at high speed Stir for 1 minute, then put into 5% cholic acid solution and stir at 400 rpm for 2 hours; collect by centrifugation and freeze-dry to obtain the final product.
Embodiment 2
[0059] 1) A mixture of 30 g of 1,6-bis(p-carboxyphenoxy)hexane in 300 ml of acetic anhydride was refluxed to form acetylated 1,6-bis(p-carboxyphenoxy)hexane;
[0060] 2) 2.7 g of compound A, 29 mg of compound B, 130 mg of dicyclohexylcarbodiimide and 5 mg of pyridine were mixed, stirred overnight at room temperature; then washed with ether and dried under vacuum to obtain a polymer;
[0061] 3) Mix the products of step 1) and step 2) into a flask, melt and polymerize under reduced pressure at 150°C for 1 hour; dissolve the polymer in chloroform after cooling to room temperature, wash with petroleum ether and dry;
[0062] 4) Put pirarubicin and the polymer into a solution mixed with 5ml of ethanol and 5ml of dichloromethane; then put it in an oven for 24 hours; at minus 10-20 degrees, ultrasonic for 2 minutes; then homogenizer Stir at high speed for 2 minutes, put the product in 3% cholic acid solution and stir at 400 rpm for 2 hours; collect by centrifugation and freeze-dry t...
Embodiment 3
[0064] 1) A mixture of 1,6-bis(p-carboxyphenoxy)hexane 17 g in 500 ml acetic anhydride is refluxed to form acetylated 1,6-bis(p-carboxyphenoxy)hexane;
[0065] 2) 1.8 g of compound A, 26 mg of compound B, 90 mg of dicyclohexylcarbodiimide and 4 mg of pyridine were mixed with 10 ml of chloroform, stirred overnight at room temperature; then washed with ether and dried under vacuum to obtain a polymer;
[0066] 3) Mix the products of step 1) and step 2) into a flask, melt and polymerize under reduced pressure at 170°C for 1 hour; dissolve the polymer in chloroform after cooling to room temperature, wash with petroleum ether and dry;
[0067] 4) Put arubicin and the polymer of step 3 into a solution mixed with 10ml of dichloromethane and 6ml of dimethyl sulfoxide; then put it in an oven to dry for 1 hour; at minus 20-30 degrees, ultrasonic 1 minute, then high-speed stirring with a homogenizer for 5 minutes, the product was put into 2% cholic acid solution and stirred at 400 rpm fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com