Pharmaceutical composition containing cavidine total alkali and preparation method of pharmaceutical composition
A technology of cavedins and compositions, applied in the field of pharmaceutical compositions, can solve the problems of poor water solubility of cavedins, inability to reach a therapeutic dose, slow disintegration of ordinary tablets, etc., and achieves good stability and portability. The effect of convenience, good compliance and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
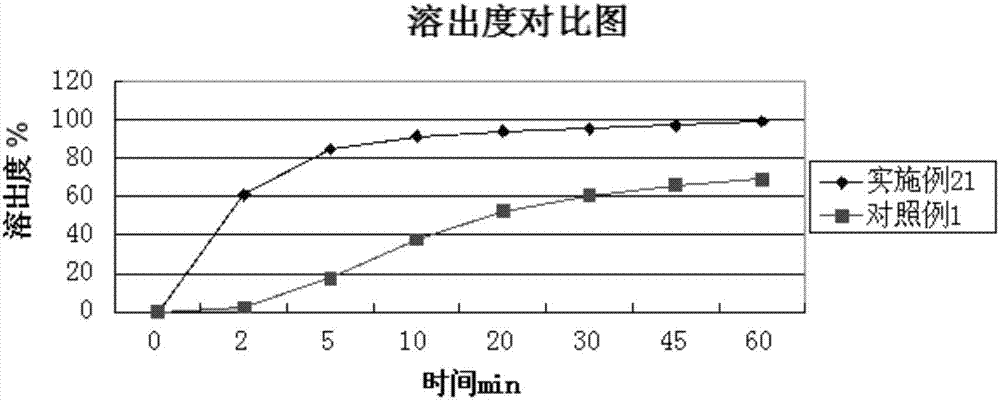
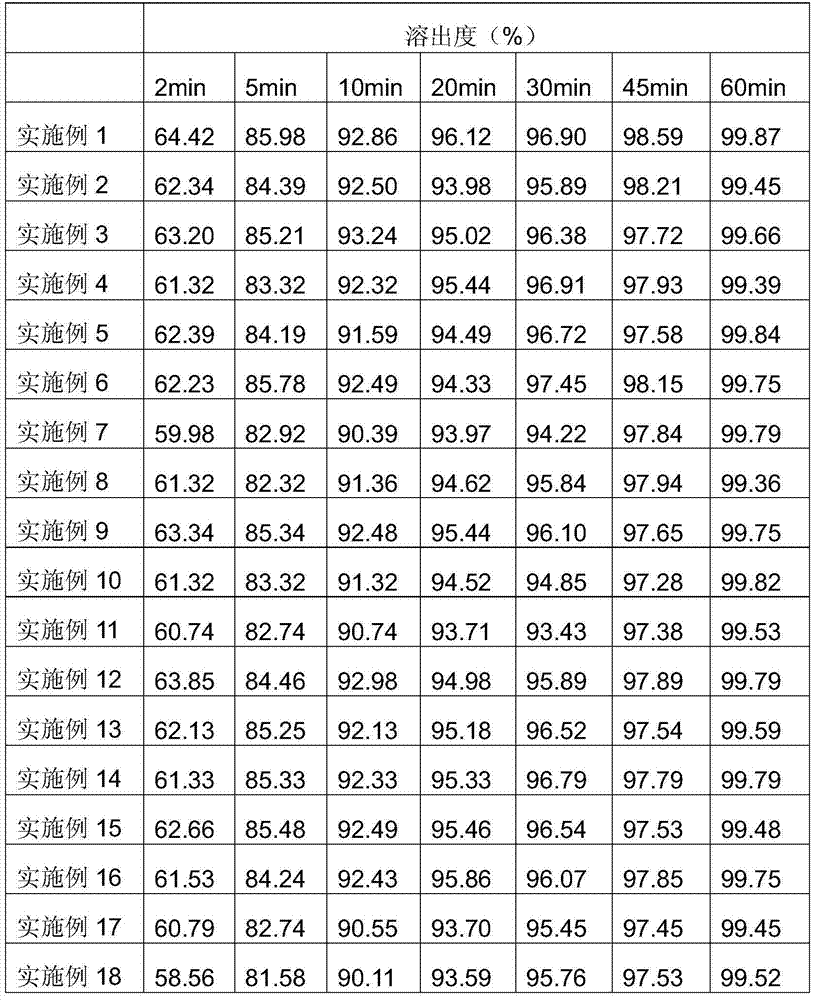
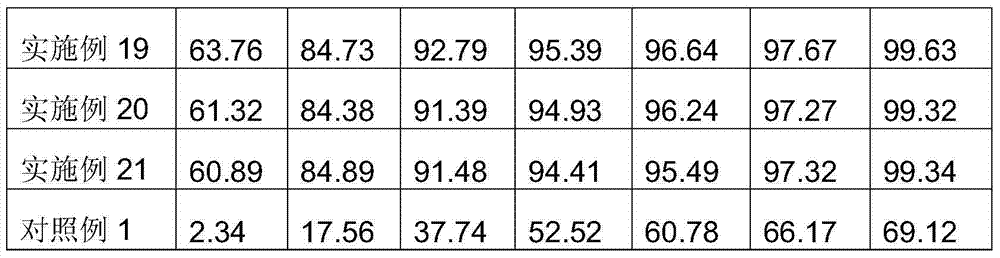
Examples
Embodiment 1
[0049] Weigh 5 mg of total alkaloids of carvedins, 15 mg of precrosslinked starch, 10 mg of microcrystalline cellulose, 0.1 mg of sodium lauryl sulfate, 5 mg of crospovidone, 1 mg of croscarmellose sodium, and an appropriate amount of sweetener. Flavoring agent, pass through 80 mesh sieve, mix evenly, add dropwise 5% polyvinylpyrrolidone aqueous solution to make soft material, pass through 24 mesh sieve to granulate, dry the granules at 45-80°C, the water content reaches 0.5-10%, granulate, Add 0.15 mg of magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0051] Weigh 5 mg of total alkaloids of carvedins, 15 mg of precross-linked starch, 10 mg of microcrystalline cellulose, 0.1 mg of sodium lauryl sulfate, 5 mg of crospovidone, 1 mg of croscarmellose sodium, and an appropriate amount of sweetener. Flavoring agent, pass through 80 mesh sieve, mix evenly, add dropwise 5% polyvinylpyrrolidone aqueous solution to make soft material, pass through 24 mesh sieve to granulate, dry the granules at 45-80°C, the water content reaches 0.5-10%, granulate, Add 0.15 mg of micropowder silica gel, mix well, and press into tablets to obtain.
Embodiment 3
[0053] Weigh 25 mg of total alkaloids of carvedins, 15 mg of pre-crossed starch, 50 mg of lactose, 0.5 mg of sodium lauryl sulfate, 5 mg of crospovidone, 10 mg of croscarmellose sodium, and an appropriate amount of sweetener, Pass through a 80-mesh sieve, mix evenly, add dropwise 5% polyvinylpyrrolidone aqueous solution to make soft material, pass through a 24-mesh sieve to granulate, dry the granules at 45-80°C, the moisture reaches 0.5-10%, granulate, add stearin Magnesium acid 1.2mg, mixed evenly, tableted.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com