Application of ginsenoside Rh1 in preparation of drugs for improving glucocorticoid resistance
A technology of glucocorticoids and ginsenosides, applied in the field of medicine, can solve the problems of less research and achieve the effects of improving hormone resistance, reducing patient pain, and improving anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
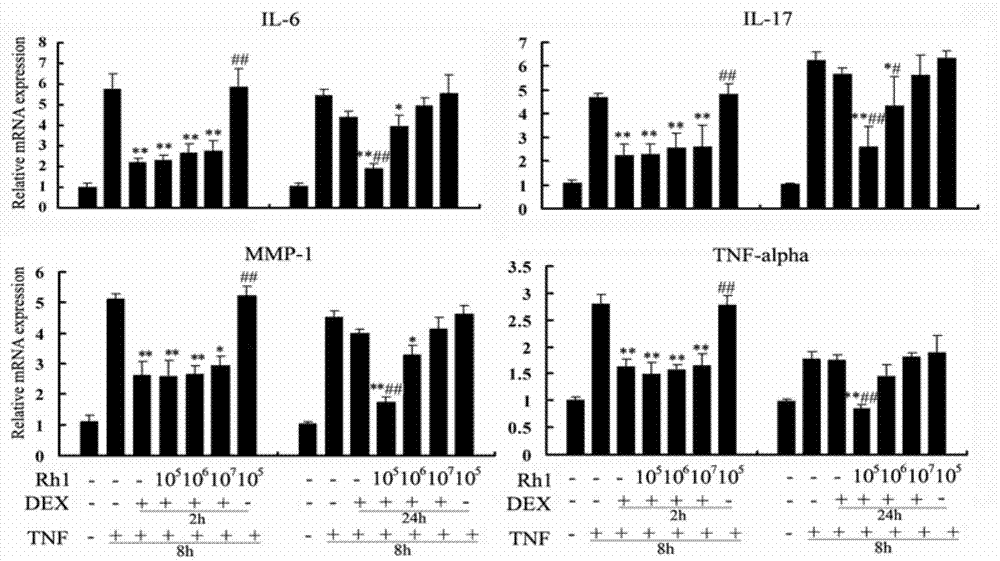
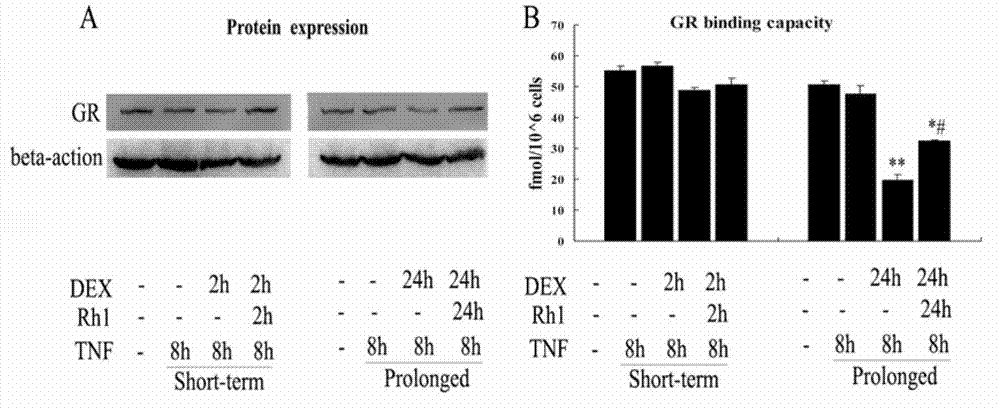
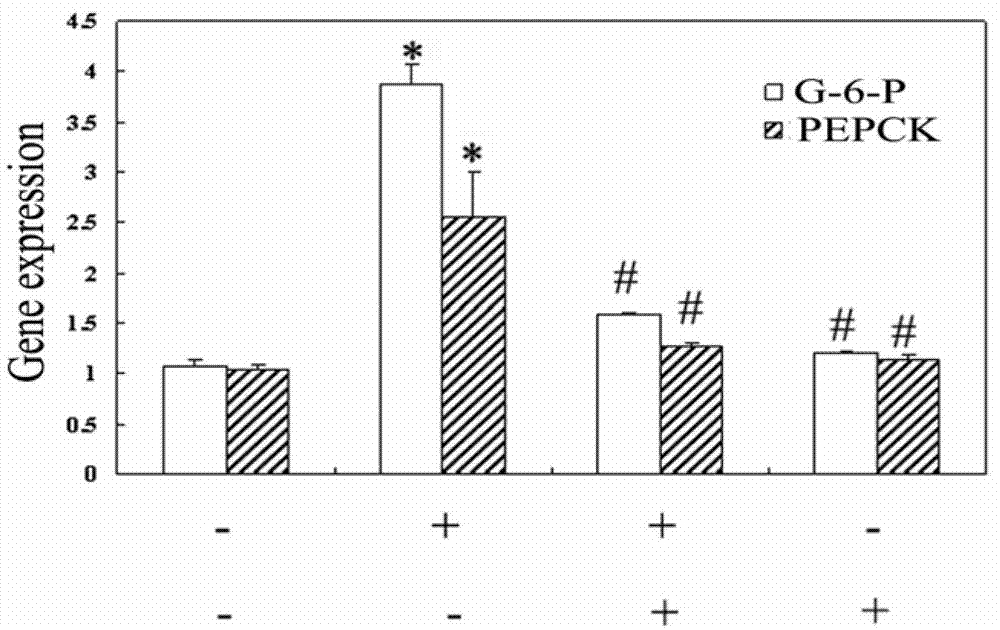
[0020] Example 1 (in vitro study of ginsenoside Rh1 improving hormone resistance and improving the anti-inflammatory effect of dexamethasone)
[0021] (1) Materials and methods
[0022] 1. Cell line: mouse monocyte-macrophage (RAW264.7), purchased from ATCC. Primary mouse hepatocytes were isolated from BALB / C mice. The above cell lines were incubated in DMEM medium containing 10% fetal bovine serum at 37°C with a volume fraction of 5% CO 2 , Routine culture under fully saturated humidity conditions, replace the medium after 48 hours, when the cell growth reaches saturation, digest with 0.25% trypsin + 0.02% EDTA for passage, pass once every 2-3 days, and use logarithmic growth phase cells in the experiment.
[0023] 2. The effect of ginsenoside Rh1 on the inflammatory response induced by TNFα.
[0024] 2 × 10 on a 24-well plate 5 and 1×10 5 RAW264.7 cells were plated, and after the cells adhered to the wall, dexamethasone / ginsenoside Rh1+dexamethasone was added (dexametha...
Embodiment 2
[0036] Example 2 (Therapeutic effect of ginsenoside Rh1 combined with dexamethasone on mouse arthritis caused by Freund's adjuvant)
[0037] (1) Materials and methods
[0038] 1. Experimental animals
[0039] DBA-1 mice, 6-8 weeks old, were provided by the Experimental Animal Center of the Second Military Medical University of the Chinese People's Liberation Army. Raised in separate cages under the condition of no special pathogenic bacteria (SPF), controlled room temperature (23±1°C), photoperiod 12 / 12, free to forage for food and drink water.
[0040] 2. Preparation of main reagents
[0041] Mixture of bovine type Ⅱ collagen and complete Freund's adjuvant: take an equal volume of bovine type Ⅱ collagen and complete Freund's adjuvant, fully mix and emulsify until it is still standing for more than 4 hours without delamination.
[0042] 3. Model preparation and group administration
[0043] Modeling: On the first day of modeling, each mouse was subcutaneously injected with...
Embodiment 3
[0048] Embodiment 3 (preparation of ginsenoside Rh1 tablet)
[0049] Take 150g of ginsenoside Rh, 10g of hydroxypropyl methylcellulose, 50g of microcrystalline cellulose, 5g of croscarmellose sodium, mix well, add an appropriate amount of 60% ethanol to make a soft material, pass through a 24-mesh sieve to granulate, Dry at 50°C for 2 hours, pass the dried granules through a 30-mesh sieve for granulation, add 2.5g of magnesium stearate, mix well, and press into 1000 tablets respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com