Preparation and application of dendritic polymer grafted with anthracycline antibiotic
A kind of polymer and dendritic technology, applied in the field of dendritic polymers, can solve the problems of low solubility, short half-life, difficult clinical application, etc., and achieve the effect of easy dissolution and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
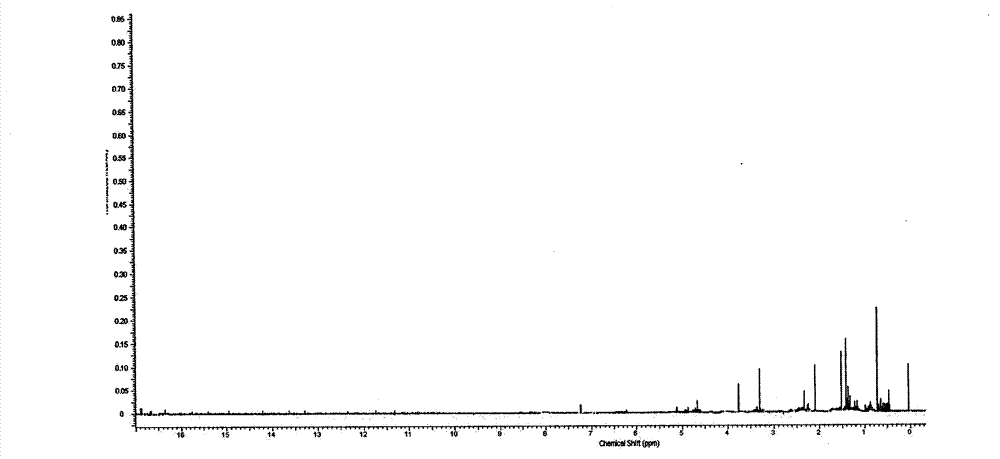
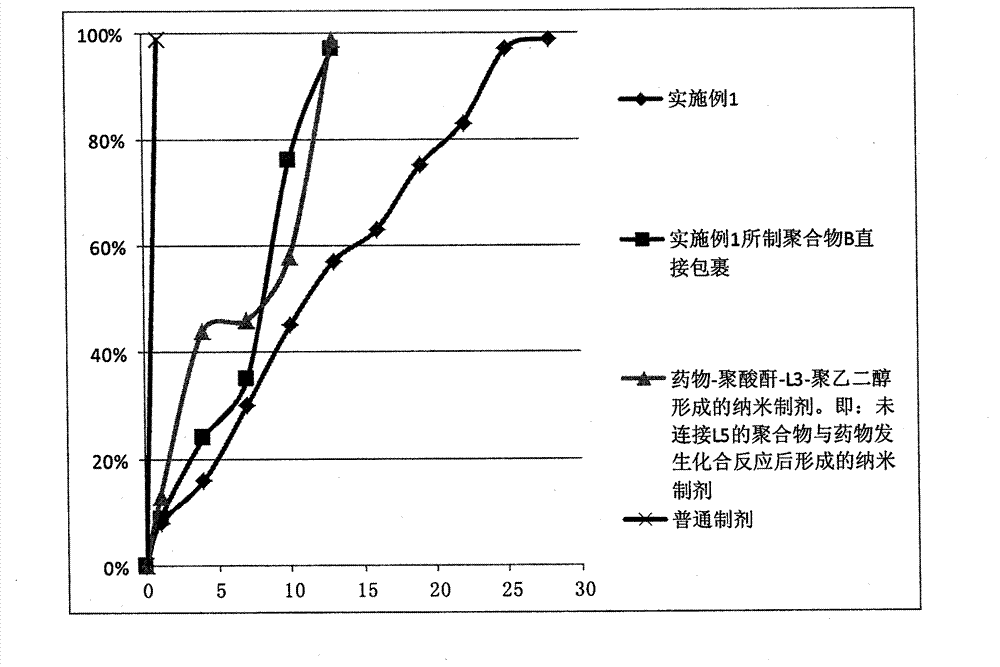
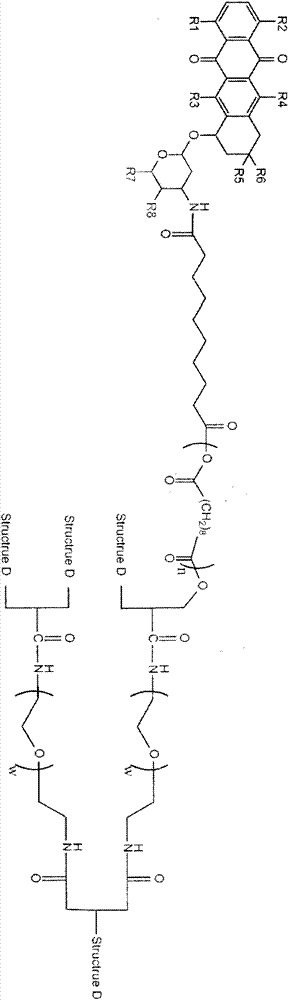
Image
Examples
Embodiment 1
[0042] 1 A mixture of 80 g of sebacic acid in 800 ml of acetic anhydride was refluxed to form acetylated sebacic acid
[0043] 2 Compound L5 containing a hydroxyl group and a polycarboxylic group: 200mg with Boc-NH-PEG-NH 2 2g is put into the flask and reacted, namely: React with L5, add 160 mg of dicyclohexylcarbodiimide and 8 mg of tetrahydrofuran at the same time, mix and add 15 ml of chloroform, and stir overnight at room temperature; then wash with ether and dry under vacuum to obtain a polyamino end with Boc protection Dendrimer A1:
[0044]
[0045] 3 Compound A1 is catalyzed by trifluoroacetic acid with unit carboxylic acid L3 containing polyalcohol After 30 mg reaction, the dendrimers A2 with polyol endings were obtained by filtration through a cellulose acetate membrane, A2:
[0046]
[0047] Mix acetyl-sebacic acid with compound A2, and react for 1 hour under reduced pressure solution polymerization (high vacuum melt polycondensation) at 175°C; after ...
Embodiment 2
[0057] A mixture of 100 g of sebacic acid in 900 ml of acetic anhydride is refluxed to form acetyl-sebacic acid;
[0058] 2 Compound L5 containing multiple carboxyl groups:
[0059] 46mg with Boc-NH-PEG-NH 2 3g was put into a flask for reaction, while 160mg of dicyclohexylcarbodiimide and 6mg of tetrahydrofuran were put in, mixed with 18ml of dichloromethane, stirred at room temperature overnight; then washed with ether, and dried under vacuum to obtain Protected polyamino-terminated dendrimers A1;
[0060] 3 Compound A1 is catalyzed by trifluoroacetic acid with unit carboxylic acid L3 containing polyalcohol After 45 mg of the reaction, filter through a cellulose acetate membrane to obtain a dendritic compound A2 with a polyol ending, mix acetyl-sebacic acid with the compound A2, and react under reduced pressure at 180°C for 1 hour; wait for the polymer to cool Dissolve with chloroform at room temperature, wash with petroleum ether and dry to obtain dendritic polymer B; ...
Embodiment 3
[0063] A mixture of 2 g of sebacic acid in 20 ml of acetic anhydride was refluxed to form acetyl-sebacic acid;
[0064] 2 Compound L5 containing multiple carboxyl groups: 300mg with Boc-NH-PEG-NH 2 Put 2.5g into a flask for reaction, put 120mg of dicyclohexylcarbodiimide and 5mg of tetrahydrofuran at the same time, mix and add 25ml of chloroform, stir overnight at room temperature; then wash with ether, and dry under vacuum to obtain The polyamino terminated dendrimer A1;
[0065] 3 Compound A1 is catalyzed by trifluoroacetic acid with unit carboxylic acid L3 containing polyalcohol After 30 mg of the reaction, filter through a cellulose acetate membrane to obtain a dendritic compound A2 with a polyol ending, mix acetyl-sebacic acid with compound A2, and react for 1 hour under reduced pressure at 175°C; wait for the polymer to cool Dissolve with chloroform at room temperature, wash with petroleum ether and dry to obtain dendritic polymer B;
[0066] 4 Put the polymer of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com