Pharmaceutical composition of microspheres for preventing diabetic foot amputation
A technology of microspheres and compositions, which can be used in drug combination, drug delivery, metabolic diseases, etc., and can solve the traumatic problems of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of a pharmaceutical composition comprising microspheres of PLGA loaded with EGF
[0036] Preparation of EGF-loaded microspheres
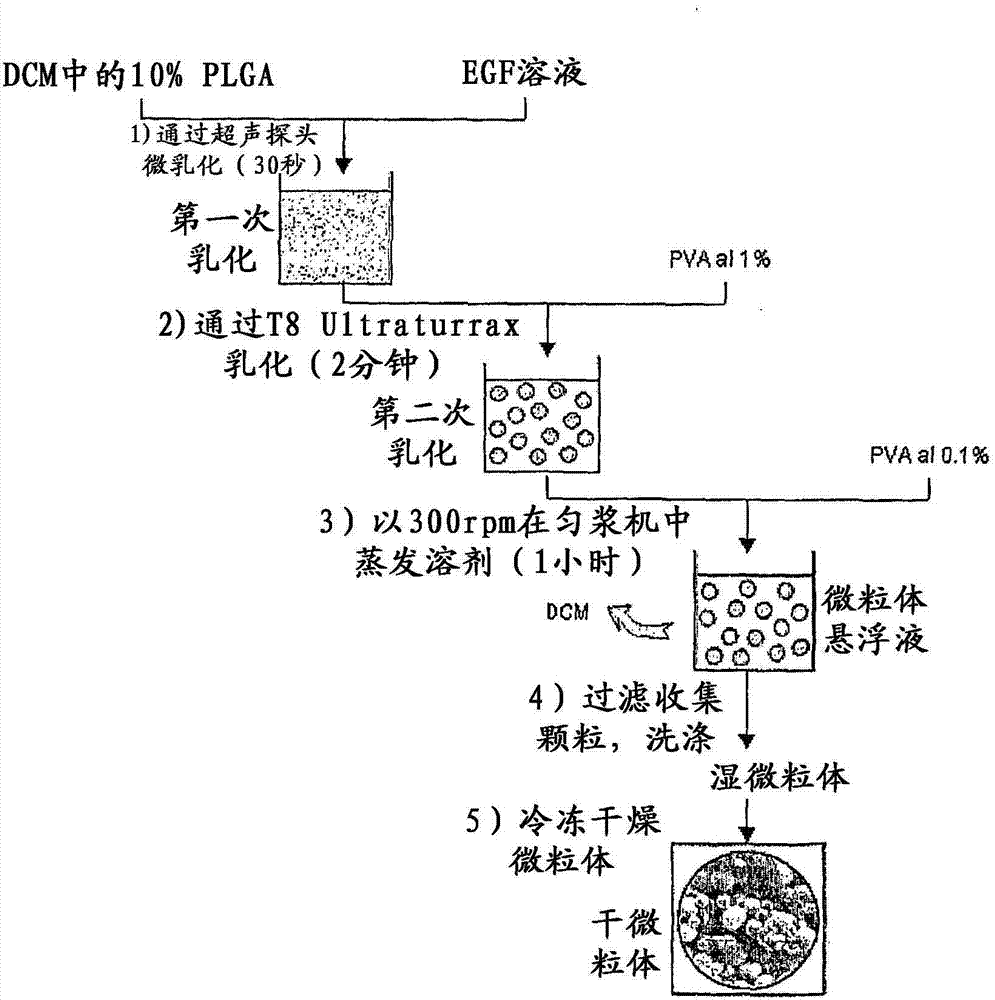
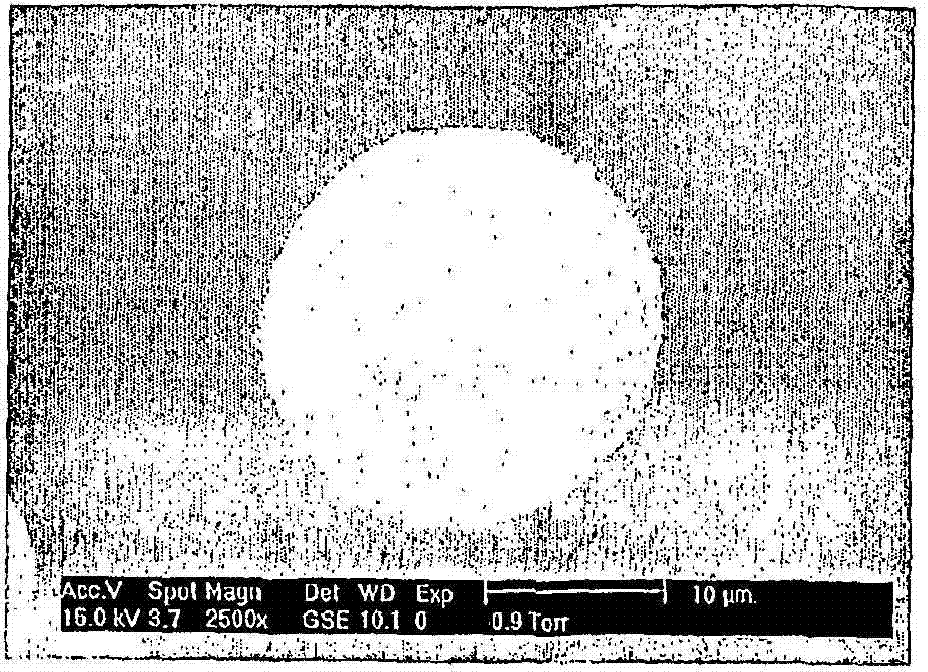
[0037] 1 g of the polymer was dissolved in dichloromethane (DCM) to make a polymer solution (PLGA50:50 (Sigma, St. Louis, Missouri, USA) 10% (w / v)). Add 1 ml of PLGA solution into the glass container, and then add 200 μl of EGF aqueous solution with a concentration of 20 mg / ml. The above-mentioned mixture was ultrasonicated for 30 seconds with an ultrasonic probe (IKASONIC U200S control (IKA Labortechnik, Germany). The first emulsion was added to 40 ml of 1% polyvinyl alcohol, and a T8 Ultraturrax (IKA Labortechnik, Germany) was used to carry out a strong 14000rpm through the opposite phase. Stir to obtain the second emulsion.Add 140ml concentration of double emulsion to 0.1% polyvinyl alcohol 30000-70000 (Sigma, St.Louis, Missouri, USA), and stir 1 hour at 300rpm with homogenizer (IKA Labortechnik, Germany) , to evap...
Embodiment 2
[0060] Example 2. In vivo effects of encapsulated EGF compared to free EGF (in animal models)
[0061] Experimental Model of Controlled Acute Trauma
[0062] The purpose of the trial described here was to evaluate the healing effect of a new pharmaceutical formulation containing EGF microspheres on acute wounds with a satisfactory prognosis, for infiltration at the wound margins and bottom or for parenteral administration by injection.
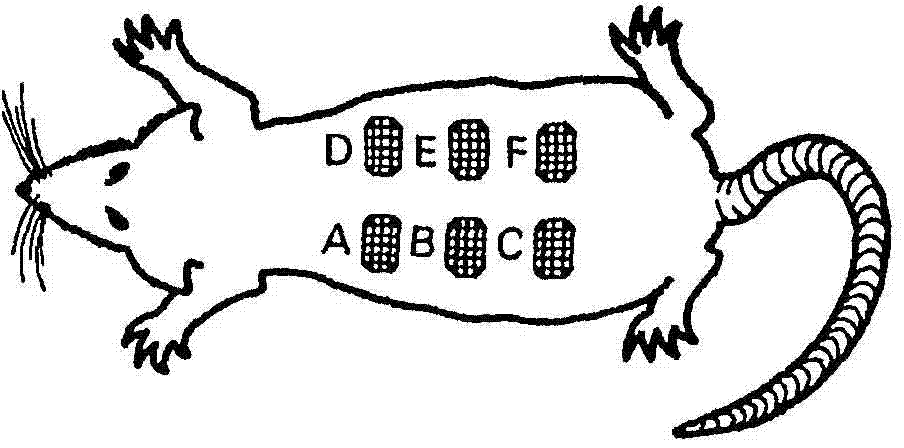
[0063] test organism model : Male Wistar rats weighing 225-250 g. Animals were housed in a controlled area of CIGB's animal facility under a constant light pattern of 12 x 12 hours, air exchange circulation, and free access to food. Rats were housed individually in T3 boxes and the bedding (pre-sterilized) was changed every 48 hours.
[0064] induced ulcer : Animals were anesthetized by intraperitoneal injection of ketamine / xylazine. The rat's back was depilated mechanically and chemically from the retroscapular space to the sacrum. ...
Embodiment 3
[0123] Example 3. In vivo effect of encapsulated EGF compared to free EGF (in patients with chronic ischemic skin ulcerative wounds)
[0124] A formulation based on EGF microspheres (without excipients) with sustained release properties was administered to patients with diabetic foot ulcers at risk of severe amputation. A 58-year-old female diabetic patient with a right foot of 30.5 cm was treated with the subject formulation of the present invention. 2 chronic ulcers and evidence of ischemia in the affected lower extremity. After wound debridement, in one month, once every 15 days, the preparation of EGF microspheres with slow-release properties was infiltrated on the edge and bottom of the wound. From the first week after starting the treatment, a rapid formation of beneficial granulation tissue was observed, which reached 100% coverage of the affected area by the third week. The patient showed satisfactory development with complete closure of the wound avoiding the need f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com